, Leonid Tiutin2 and Thomas Schwarz3
(1)
Russian Research Center for Radiology and Surgery, St. Petersburg, Russia
(2)
Department of Radiology and Nuclear Medicine, Russian Research Center for Radiology, St. Petersburg, Russia
(3)
Department of Nuclear Medicine Division of Radiology, Medical University Graz, Graz, Austria
Abstract
Currently it is accepted to distinguish among lymphoproliferative diseases the groupings of lymphomas, leukoses and lymphomas/leukoses (Gantsev 2004). Malignant neoplasms originating from the elements of lymph nodes or extranodal lymphatic tissue are qualified as lymphomas, while leukoses are tumors from peripheric blood cells, and the group of lymphomas-leukoses consists of diseases with both signs. Neoplasms of plasma cells, including myelomatosis and plasmocytoma, are not presently qualified as lymphomas.
Currently it is accepted to distinguish among lymphoproliferative diseases the groupings of lymphomas, leukoses and lymphomas/leukoses (Gantsev 2004). Malignant neoplasms originating from the elements of lymph nodes or extranodal lymphatic tissue are qualified as lymphomas, while leukoses are tumors from peripheric blood cells, and the group of lymphomas-leukoses consists of diseases with both signs. Neoplasms of plasma cells, including myelomatosis and plasmocytoma, are not presently qualified as lymphomas.
Malignant lymphomas (MLs) are the most frequent form of lymphoproliferative diseases. Presently MLs constitute 5% of registered cases in oncologic morbidity. However, in recent years, a 3.9-times increase in this index has been observed. In most cases, MLs occur at the age of 16–40 years and have a chronic course. The disease is found in all countries, but currently some geographic regularities in epidemiology of certain kinds of lymphomas are established. For example, extranodal lymphomas localized in the oropharyngeal area are most often detected in Italy, primary lymphomas of the gastrointestinal tract are diagnosed in the Middle East. Endemic regions in equatorial Africa are described in which the biggest number of cases of Burkitt’s lymphoma are registered (Gantsev 2004).
Statistical data of recent years show that 55–60% of ML cases are lymphogranulomatoses [Hodgkin’s disease or Hodgkin’s lymphoma (HL)]. HL is a primary tumor disease of lymphatic tissue, occurring unicentrally and disseminating by way of metastasis. The tumor substrate in HL, unlike other malignant neoplasms, consists of mature differentiated cells. Pathologic cells in the lymph node are clearly in the minority and are represented by Reed-Sternberg-Berezovsky giant cells, which are not diagnostically significant. The cellular composition includes both B-lymphocytes in different stages of maturation and T-lymphocytes with phenotype of T-helpers and T-supressors. Such heterogeneity of tumor dictates the necessity of carrying out morphologic and immunohistochemical study of material, which permits detection of Reed-Sternberg-Berezovsky giant cells (Vazhenin et al. 2003).
Lymphoprolipherative disease may develop in any organ or tissue where there are lymphoid cells (lymphoblasts, lymphocytes, follicular center cells – centrocytes and centroblasts). Normally lymphoid cells are contained in lymph nodes, in the lymphatic pharyngeal ring (palatine tonsils, tonsils of the tongue, adenoids), thymus, aggregated lymphatic follicles (Peyer’s plaques) of the small intestine, spleen and in extralymphatic organs (Gershanovich et al. 1995). The latter include brain, spinal cord, bone marrow, lungs, gonads, kidneys, gastrointestinal tract, skin, bones, uterus and tissues surrounding the eye-bulb (eye conjunctiva, lacrimal glands and soft tissues of the eye-socket).
The scientific literature indicates that considerable difficulty has long existed in drawing up the optimum classification of lymphoprolipherative diseases which would satisfy both clinicians and pathomorphologists. However, the rapid development of molecular-genetic, immunomorphologic and biochemical methods of examination has facilitated the elaboration of a unified classification of lymphoid tumors (Table 16.1) by the experts of the World Health Organization (WHO). The assessment of tumor dissemination is done according to the Ann Arbor classification. It reflects the anatomic situation of pathologic foci relative to the diaphragm, number of affected regions, and the tumor affection of extralymphatic organs. Besides, in staging of the disease based on anamnestic data and on the results of physical examination, intoxication symptoms (fever, night pouring perspiration, loss of weight) are obligatorily taken into account, which permit to qualify a given case as belonging to A or B subgroups.
Table 16.1
WHO classification of the main forms of lymphoid neoplasms (1999)
Morphological and immunological tumor type | Nosological form |
|---|---|
B-cell precursor tumors | •B-lymphoblastic lymphoma (acute B-cell lymphoblastic leukemia) |
peripheric (mature) B – cell tumors | •Chronic B-cell lymphocytic leukemia (small lymphocyte lymphoma) |
•Plasma cell myeloma (plasmocytoma) | |
•MALT extranodal B-cell lymphoma of the marginal area | |
•Follicular lymphoma | |
•Mantle cell lymphoma | |
•Diffuse B-large cell lymphoma | |
•Burkitt lymphoma | |
T – cell precursor tumors | •Acute T-cell precursor lymphoblastic leukemia |
Peripheric (mature) T-cell tumors | •T-cell prolymphocytic leukemia |
•T-cell large-granular lymphocyte leukemia | |
•Aggressive NK-cell leukemia | |
•T-cell lymphoma (adult leukemia) | |
•Extranodal T-cell lymphoma, nasal type | |
•T-cell lymphoma with enteropathy | |
•Hepatolienal T-cell lymphoma | |
•T-cell panniculitis-like lymphoma of subcutaneous tissue | |
•Mycosis fungoides | |
•Peripheric T-cell lymphoma, unspecified | |
•Angioimmunoblastic T-cell lymphoma | |
•Anaplastic large-cell lymphoma with primary systemic lesion | |
Hodgkin’s lymphoma | •Lymphoid predominance |
•Nodular sclerosis | |
•Mixed-cell variant | |
•Lymphoid exhaustion |
16.1 Methods of Diagnosis
The WHO has elaborated and endorsed an algorithm for examining patients with lymphoproliferative diseases, including obligatory and auxiliary diagnostic procedures. The obligatory procedures are: morphological study of the biopsy material, laboratory studies, X-ray of the organs of the thorax, CT of the thoracic and abdominal cavities and of the organs of the small pelvis. Additionally, all the patients undergo trepan biopsy. The auxiliary procedures include laparotomy with splenectomy, puncture biopsy of the liver, scintigraphy of the skeleton in case of marked pain syndrome in the bones, CT of the head and neck organs, gastroscopy or X-ray of the organs of the abdominal cavity in patients with suspected lesion of the gastrointestinal tract, MRI of the spine and cytological study of cerebrospinal fluid in patients with the fourth stage of the disease and meningeal symptoms (Gantsev 2004).
16.1.1 PET Diagnosis
Staging of Lymphoproliferative Diseases
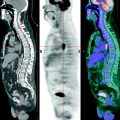
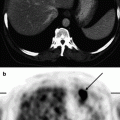
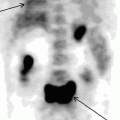
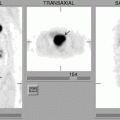
The role 18F-FDG PET in diagnosing lymphoproliferative diseases is very important. The method is widely used in staging of the tumor process, treatment efficiency evaluation, including early stages of therapy, making prognosis, radiotherapy planning and diagnosis of relapses (Buchmann et al. 2001; Carr et al. 1998; Fuster et al. 2006). The obtaining of a whole-body image in PET is the main advantage of the method. Within a single examination it is possible to assess lymph nodes of virtually all the anatomic regions as well as spleen and simultaneously to analyze the state of extralymphatic organs (Fig. 16.1). In this process the size of lymph node does not have importance. Meanwhile, the assessment of diagnostic accuracy of the method is in some cases difficult due to the absence of a “gold standard” to which visualizing abilities of PET could be compared. As a result, some pathological foci still remain without tissue verification (Aboizied et al. 2005; Barnngton and O’Doherty 2003). In some cases dynamic observation of patients may help find tumor foci detected in PET. If changes detected in PET are of great significance in determining the stage of disease, retrospective data revision obtained by other methods of radiological visualization. Such an approach can ensure correct staging and in 25–40% of cases it can permit restaging of the disease (Schiepers et al. 2003).
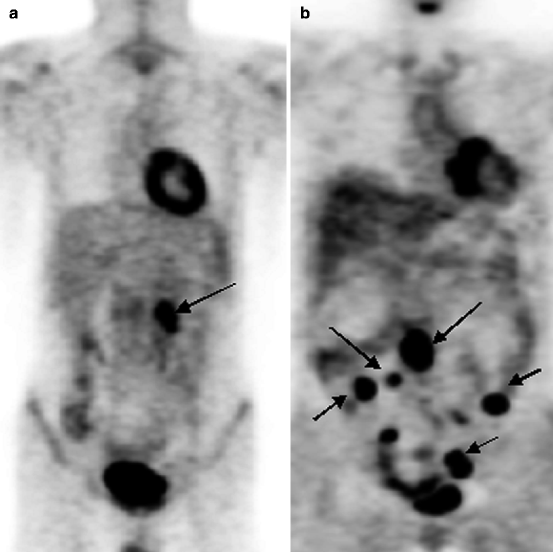
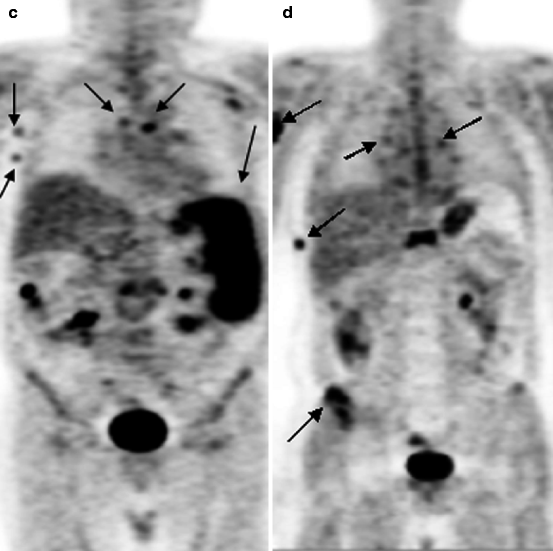


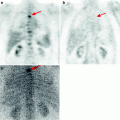
Fig. 16.1
18F-FDG PET of patients with various stages of LGM and NHL. (a) solitary pathological uptake of the retroperitoneal lymph nodes; (b) pathological uptake of intra- and retroperitoneal lymph nodes; (c) pathological uptake of mediastinal and right axillary lymph nodes and of the spleen; (d) pathological uptake of mediastinal lymph nodes, of the right humeral bone, of Th11, rib and right iliac bone
According to different authors, PET sensitivity may reach 100% in diffuse B-cell lymphoma, 98% in Hodgkin’s lymphoma and follicular lymphoma, 67% in lymphomas of the pallium area, 40% in peripheric T-cell lymphoma 95% in myelomatosis. In MALT lymphomas of the gastrointestinal tract this value usually does not exceed 57–64% (Beal et al. 2005; Buchmann et al. 2001; Elstrom et al. 2003; Fnedberg et al. 2004; Gambhir et al. 2001; Jerusalem et al. 1999). According to Schiepers (2003), such data spread of the index of sensitivity in diagnosing different morphological types of lymphomas may be conditioned by the histological type of neoplasm and degree of malignancy of tumor cells (grade). However, some research results contradict this statement. For example, Schoder et al. (2005) compared standardized uptake values (SUVs) in indolent and aggressive non-Hodgkin lymphomas (NHLs). According to them, the SUVs were significantly higher in aggressive lymphomas. At the same time, PET sensitivities for lymphomas with indolent or aggressive courses differed only slightly and were 81% and 83% respectively. In this context the authors think that SUVs do not influence the sensitivity of the method. At the same time, the specificity of PET data in the presence of high SUVs grow significantly. These results are confirmed by other researchers who also ascertained high sensitivity of the method with indolent NHL. In particular, they observed a high 18F-FDG uptake in malignant cells of follicular lymphoma, which in most cases is characterized by relatively benign course (Blum et al. 2003; Jerusalem and Beguin 2002, 2005).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree