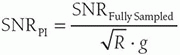Magnetic Resonance Imaging: Advanced Image Acquisition Methods, Artifacts, Spectroscopy, Quality Control, Siting, Bioeffects, and Safety
Magnetic Resonance Imaging: Advanced Image Acquisition Methods, Artifacts, Spectroscopy, Quality Control, Siting, Bioeffects, and Safety
13.0 INTRODUCTION
Advanced pulse sequences and fast image acquisition methods; methods for perfusion, diffusion, and angiography imaging; spectroscopy; image quality metrics; common artifacts; MR siting; and MR safety issues are described and discussed with respect to the underlying physics.
13.1 IMAGE ACQUISITION TIME
1. Acquisition time, 2D acquisition
a. Time = TR × #PEG × NEX—where TR is the repetition time, #PEG is the number of phase-encode gradient applications, and NEX is the number of excitations (averages).
b. Matrix size defining k-space is often not square—typically, the smaller dimension is assigned to PEG.
c. Tradeoff of time and SNR are considered.
2. Multislice data acquisition
a. During the TR, cycling gradients and tuning RF excitation frequency images the volume (
Fig. 13-1).
b. Tradeoff is cross excitation of adjacent tissues and loss of contrast from nonsquare excitation pulses.
c. Total number of slices = TR/(TE + C), where C is a constant dependent on MR equipment capabilities.
d. Longer TR (e.g., T2-weighted SE) can have more slices acquired in the acquisition.
3. Acquisition time, 3D acquisition
13.2 FAST IMAGING TECHNIQUES
13.3 SIGNAL FROM FLOW
1. Flow-related enhancement
a. A process causing increased signal enhancement of moving tissue (blood, CSF).
b. High intensity is caused by wash-in of unsaturated protons into a partially saturated volume.
c. Elimination of bright signals can be achieved with saturation pulses outside of the imaging volume.
2. MR angiography—exploitation of blood flow enhancement
3. Gradient moment nulling (for flow compensation)
13.4 PERFUSION AND DIFFUSION CONTRAST IMAGING
Perfusion is the delivery of blood to a capillary bed in tissue and permits the delivery of oxygen and nutrients to the cells and removal of waste (
e.g., CO
2) from the cells—methods include ASL, DSC, DCE (
Table 13-1).
1. Arterial spin labeling (ASL)
a. ASL uses blood magnetization and measures blood flow (
Fig. 13-12).
b. Pulsed ASL tags blood with inversion or saturation pulse.
(i) Tagged blood moves into region of interest with imaging initiated.
(ii) After waiting time (1 to 2 s), another image set without inversion is acquired in same region.
(iii) Subtraction of the images removes the signals from static tissues.
c. Continuous ASL uses a long RF pulse applied in a plane while the blood signal is inverted.
d. Method suffers from low SNR, requiring multiple signal averages (20 to 60).
e. Most important is choice of waiting time—a tradeoff of measuring vascular signals versus tissue perfusion and the T1 decay of blood signals.
2. Dynamic susceptibility contrast (DSC)
a. Use of contrast material tagged with susceptibility agents such as gadolinium (Gd).
b. T2 and T2
* parameters generate large signal differences in the vasculature—Gd concentrations can be tracked as a function of time in arteries and tissues (
Fig. 13-13).
c. Blood flow can be estimated by deconvolution of the tissue residue function (
Fig. 13-14).
d. Concern with DSC is contrast agent leakage to extravascular or extracellular space, as the conventional DSC model is based on no leakage.
3. Dynamic contrast enhanced (DCE)
a. Purpose is the measure the amount of leakage into tissues.
b. “Tofts” and “Extended Tofts” models are used (see textbook Pg. 515-516 for details).
c. Dynamic T1-weighted sequence such as SPGR is used.
d. Mapping of T1 signal change when Gd contrast agent is injected (
Fig. 13-15).
4. Diffusion MRI
Molecular diffusion is the stochastic translational motion of molecules also known as Brownian motion. Diffusion MRI sequences use strong MR gradients applied symmetrically about the refocusing pulse to produce signal differences based on the mobility and directionality of water diffusion.
13.5 OTHER ADVANCED TECHNIQUES
1. Functional MRI
a. Technique of mapping neuronal activities in the brain and relying on a local reduction of deoxyhemoglobin, which is paramagnetic, while the neurons in the region become active
b. Blood oxygen level-dependent (BOLD) acquisition
(i) A series of dynamic T2*-weighted images using fast EPI GRE sequences map the brain’s active regions through correlation of signals with a repetitive task (e.g., physical, sensory, or cognitive).
(ii) Resultant areas are color mapped onto the gray scale images (see textbook, Pg. 521,
Fig. 13-26).
c. Combination diffusion MRI and fMRI can help visualize structure and function (
e.g., identify areas prior to resection of a brain tumor) (see textbook, Pg. 521,
Fig. 13-27).
2. Susceptibility-weighted imaging
a. Based on T2*-weighted magnitude and phase images obtained with a 3D GRE sequence.
b. Images are sensitive to local susceptibility differences from deoxyhemoglobin in venous blood, methemoglobin in blood hemorrhage, and iron deposition.
c. Technique utilizes small and local phase differences and magnitude change with image processing to remove phase changes due to field inhomogeneities.
d. SWI images are generated from a multiplication of phase masks with the magnitude images to emphasize small phase deviations caused by the susceptibility agents (
Fig. 13-19).
e. Improved visualization is obtained with minimum intensity projection (mIP) of the SWI image stack.
3. MR elastography
a. Technique evaluates the stiffness of tissues with the use of an external mechanical wave generator.
b. GRE imaging with motion-encoding gradients (similar to phase-contrast MRA) are used for acquisition.
(i) 4 to 8 dynamic images are acquired of magnitude and phase during mechanical vibration.
(ii) Displacement information is extracted from the dynamic phase information at the applied frequency.
(iii) Submicrometer motion in the direction of mechanical wave is inversely proportional to the wave speed, from which the stiffness is determined, as stiffer tissues have faster wave speed.
c. Typical use is assessment of tissue stiffness in the liver (
Fig. 13-20).
4. Magnetization transfer contrast
a. The interaction between protons in free water molecules and protons hydrated to the macromolecules of a protein provides a conduit for partial saturation with off-resonance pulse.
b. Magnetization exchange occurs between the two proton groups.
c. Selective saturation of the protons in the hydration layer occurs separately from the bulk water by using narrowband RF pulses tuned to the hydration layer with an off-resonance peak of approximately 5 kHz.
d. Transfer of the magnetization partially saturates the adjacent protons in bulk water.
e. Saturation of these spins reduces anatomic contrast and is often used in conjunction with MRA time-of-flight methods and other examinations where selective reduction of high signal assists in diagnosis, such as knee cartilage (see textbook, Pg. 522-523,
Fig. 13-30).
5. Magnetic resonance spectroscopy (MRS)
a. A method to evaluate tissue chemistry by recording and measuring signals from metabolites.
b. MRS metabolic peaks are separable by frequency shifts relative to the frequency of water proton.
c. Metabolite precessional frequencies are bounded by that of water and fat requiring selective saturation pulses to allow the quantitative evaluation of the very weak metabolite signals (
Fig. 13-21).
(i) CHESS (Chemical Shift-Selective) or STIR (
Chapter 12) signal suppression methods are used.
d. MRS signals are derived from proton metabolites in targeted tissues (
Fig. 13-22,
left, and
Table 13-2).
e. Localization of the target volume is achieved with single voxel or multivoxel techniques.
(i) Single voxel sampling areas are about 1 cm3—a STEAM (STimulated Echo Acquisition Mode) or a PRESS (Point REsolved SpectroScopy) sequence are employed to identify the volume.
(ii) Multivoxel MRS uses a CSI (chemical shift imaging) technique to delineate multiple voxels of approximately 1 cm3 in 1, 2, or 3 planes over a block of several centimeters of tissue.
f. Magnetic resonance spectroscopic imaging (MRSI) generates the signal intensity of a single metabolite in each voxel and color encodes the values (
e.g., the choline/creatine ratio), which are superimposed as a map on the anatomical MR image (
Fig. 13-22,
right) indicating normal/abnormal tissues (
Table 13-3).

 F. 13-31
F. 13-31

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access