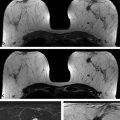
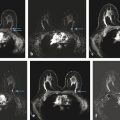
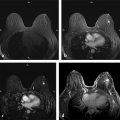
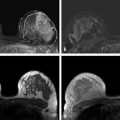
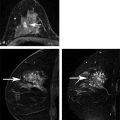
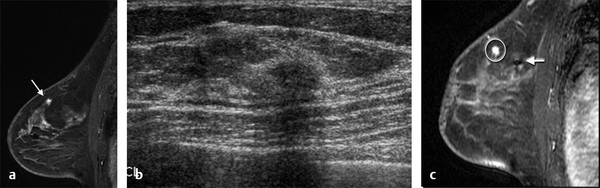
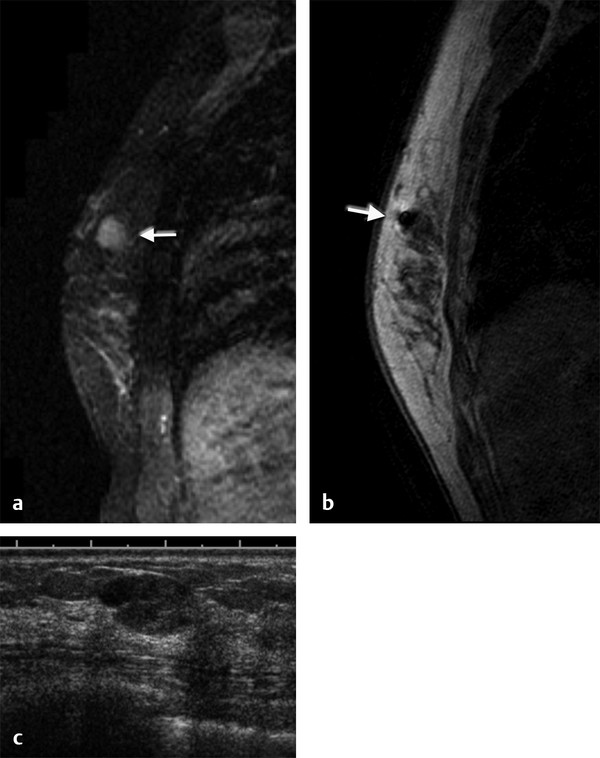
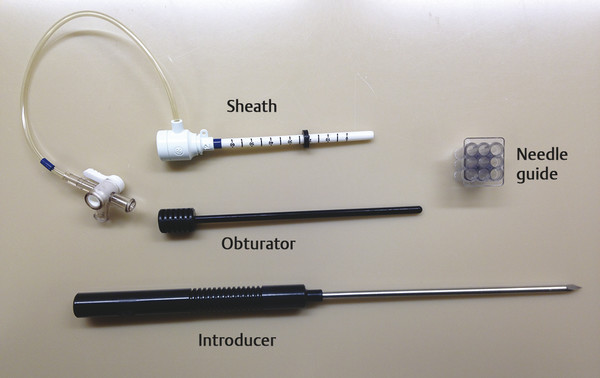
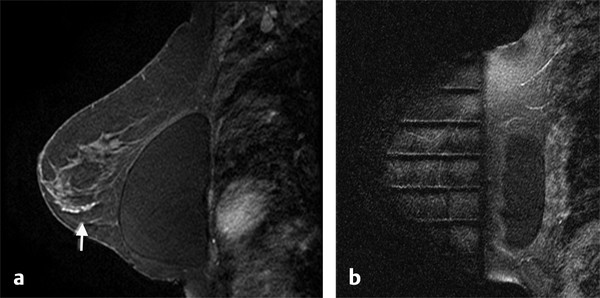
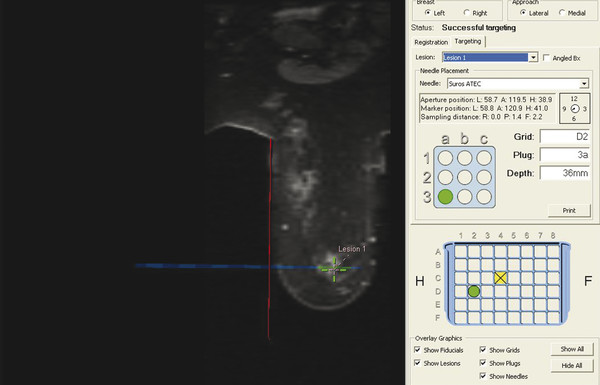
Positive predictive value for malignancy at MRI-guided core biopsy Author (year) No. of lesions Needle gauge Cancers Perlet et al (2002) 2 334 11 84 (25%) Orel et al (2006) 3 85 9 52 (61%) Liberman et al (2007) 29 98 9 24 (25%) Perlet et al (2006) 5 517 11 138 (27%) Mahoney (2008) 6 55 10 10 (18%) Han et al (2008) 7 150 9 or 10 43 (29%) Rauch et al (2012) 8 218 9 138 (27%) Manion et al (2014) 9 445 Not stated 94 (21%) The proportion of cases that require MRI-guided biopsy will vary according to the patient population being screened, whether exams are largely prevalence or incidence exams, and the interpretation criteria. When a suspicious finding is seen on breast MRI, a recent mammogram should be reviewed to determine if a possible mammographic correlate is present. Occasionally, faint calcifications or a subtle asymmetry can be seen in retrospect that will allow a stereotactic biopsy to be done for the MR finding. If the mammogram is negative, targeted ultrasound can be performed in an attempt to find a sonographic correlate and perform the biopsy under ultrasound guidance, even if a preceding survey ultrasound was negative. Ultrasound-guided biopsies have a number of advantages over MRI-guided biopsies including being faster, more comfortable, and less expensive. A number of studies have evaluated the success of targeted ultrasound in finding a correlate and the reported percentage varies from 23 to 67%. 10, 11, 12, 13, 14 In a study of 519 patients with suspicious MRI findings by Meissnitzer et al, a sonographic correlate was found in 56% of cases. 12 Ultrasound was more successful in finding a correlate for masses than for nonmass enhancement. Among masses, 62% were identified with ultrasound, whereas only 31% of nonmass enhancement was found and for nonmass enhancement measuring 10 mm or less, a sonographic correlate was found in only 13%. Other factors that increased the likelihood of finding a sonographic correlate were increased size and malignant versus benign histology. The information as to which lesions are most likely to have an ultrasound correlate can help guide which MR-detected lesions are best managed by going directly to MRI-guided biopsy. The results of a number of studies on targeted ultrasound after MRI are summarized in ▶ Table 8.2. Whereas finding a sonographic correlate to an MR finding is often straightforward, occasionally the lesion seen on MR and the lesion found on targeted ultrasound is not the same. When performing the targeted ultrasound, care should be taken to ensure the quadrant, size, shape, and appearance of the lesion as well as the distance from the nipple are similar between the two studies. In addition, a clip should always be placed at the time of sonographic biopsy. If the results of the biopsy are benign and felt to be concordant, a follow-up MRI should be performed to confirm correlation between the two findings. In the study by Meissnitzer et al, of 80 cases of benign, concordant ultrasound-guided biopsy for an MR finding, the two lesions did not correlate in 10 (12.5%) and 5 proved to be cancers ( ▶ Fig. 8.1). 12 In another study of 218 ultrasound-guided biopsies done for MR findings, the false-negative rate was 26% because of the lack of concordance between the two examinations. 15 Both of these studies emphasize the importance of careful correlation between ultrasound and MR findings and follow-up MR when biopsy results are benign and concordant in order to ensure accurate sampling of the lesion. The optimal timing of the follow-up study has not been fully established. A case report suggested a strategy for correlation whereby a single noncontrast, non–fat-suppressed sequence is obtained immediately after the ultrasound-guided biopsy. 16 The follow-up MRI should take just a few minutes to perform and the signal void from the clip placed at the time of biopsy should be readily apparent and can confirm accurate sampling. However, if the lesion is surrounded by glandular or fibrous tissue rather than fat, correlation may be difficult without the administration of contrast ( ▶ Fig. 8.2). Depending on availability of the magnet, an immediate postbiopsy may not be logistically feasible. Also, it is unlikely that any reimbursement could be obtained for this single sequence. For most cases, doing a 6- to 12-month follow-up MRI for benign concordant ultrasound-guided biopsies for a suspicious finding on MR may be sufficient. Fig. 8.1 Ultrasound–magnetic resonance imaging (MRI) correlation. (a) Showing sagittal postcontrast T1-weighted MRI showing 5-mm enhancing mass found on screening MRI (arrow). Targeted ultrasound revealed a hypoechoic mass thought to represent a sonographic correlate to the MR finding (b). Ultrasound-guided biopsy showed fibroadenomatoid change that was felt to be concordant. On follow-up MRI 6-months later (c), the signal void from the biopsy marker placed at the time of ultrasound biopsy (arrow) did not correspond to the original mass (circled) that was still present and larger. Subsequent MRI-guided biopsy revealed invasive ductal carcinoma. Fig. 8.2 Immediate postbiopsy imaging for concordance. Enhancing mass found on sagittal T1-weighted image (arrow, a). Targeted ultrasound showed mass that was thought to represent the correlate. Ultrasound-guided biopsy was performed and a clip placed (b). T1-weighted sequence without fat suppression done immediately after the ultrasound biopsy (c) shows the signal void from the biopsy marker clip (arrow) within the mass, confirming that the ultrasound finding was indeed the correlate. Author (year) N Seen on US: total seen on Us: malignanta Not seen on US: total Not seen on US: malignanta LaTrenta et al (2003) 10 93 21 (23%) 9 (43%) 55 (77%) 10(14%) DeMartini et al (2009) 11 167 76 (46%) 27 (36%) 91 (54%) 20 (20%) Meissnitzer et al (2009) 12 519 290 (56%) 87 (34%) 229 (44%) 34 (19%) Abe et al (2010) 13 202 115 (51%) 33 (28%) 87 (49%) 11 (13%) Destounis et al (2009) 14 182 1280 (70%) 39 (32%) 54 (30%) 8 (16%) aPercentage of malignant of those that underwent biopsy (positive predictive value [PPV3]). MRI-guided breast biopsies can be safely performed on either 1.5- or 3-T magnets and do not have to be done on the same field strength magnet used for the AB-MR examination. MRI-guided biopsies require devices that allow targeting of the lesion in the breast. The two types of devices are the compression grid and the pillar-and-post apparatus. These devices add on to the breast coil and are used to allow needle insertion into the appropriate location in the breast. Most allow access to the lateral and the medial surface of the breast simultaneously, allowing biopsy of multiple sites in a single breast. Some coils also allow simultaneous access to the lateral aspects of both breasts, allowing bilateral biopsies. If there are bilateral lesions and one or both must be accessed from a medial approach, bilateral biopsies at the same time are not possible and must be done on separate visits. Any of a variety of needles can be used with the targeting devices. Most are vacuum assisted and range from 7 to 14 gauge, though the most commonly used are 9 to 11 gauge. Some have automatic collection of the core specimen in a collection chamber; others require manual retrieval of the cores. All of the MRI needle devices have similar components. There is usually a nonmetallic sheath with centimeter markings that allows the lesion depth to be set, a sharp introducer that fits through the sheath, a needle guide, and a plastic obturator that allows scanning to confirm accurate location of the sheath ( ▶ Fig. 8.3). The actual sampling needle is then advanced through the sheath for tissue acquisition. As with any piece of equipment, the choice of which device and which needle to use is a matter of individual preference. The reported time required to perform a single site MRI-guided biopsy is between 35 and 58 minutes. 4, 17, 18 Noroozian et al evaluated the time required for MRI-guided biopsies as a function of patient, lesion, and technical variables. 18 They reported that factors such as patient age, breast size, lesion size, mass versus nonmass enhancement, or lesion location were not associated with the length of time of the procedure. The only factor that affected procedure time was the presence of a breast imaging fellow in training which decreased duration of the biopsy. Despite the fact that image acquisition time is faster with 3-T magnets, they found no significant difference in procedure time for those done on 3-T compared to 1.5-T magnets. Multiple sites and bilateral lesions will of course require more time. Liberman et al reported the average time of biopsy for a single lesion to be 35 and 65 minutes for two lesions. 4 Lehman et al had a mean time of 59 minutes when multiple lesions in one breast were biopsied and 64 minutes when bilateral biopsies were done. 17 For a single lesion, scheduling 1 hour of magnet time should be sufficient. Because the time of lesion visualization is limited due to washout and enhancement of surrounding parenchyma over time, it is advisable to be thoroughly familiar with the location and appearance of the lesion and to plan the approach prior to the procedure. During a full MR examination, a sequence in the plane orthogonal to the plane of acquisition of the dynamic series is often obtained. For example, if the postcontrast sequences are obtained in the axial plane, a single sagittal sequence is sometimes performed as the last sequence allowing visualization of a lesion in two planes. With the abbreviated protocol, imaging in only one scan plane is acquired and therefore, it may be advisable to reformat in the orthogonal plane prior to the biopsy for optimal procedure planning. This is particularly important if the biopsy is performed manually, without the aid of computer targeting software. Fig. 8.3 Typical needle biopsy setup. Informed consent should obviously be obtained prior to all procedures. In speaking with the patient, the risk of bleeding and hematoma formation should be discussed. Another potential complication is infection, which can occur days or weeks after the procedure. It is not customary to administer prophylactic antibiotics prior to breast biopsy procedures in general. Fortunately, serious complications from core biopsy procedures in general are relatively rare. There are very few reported cases of complications secondary to MRI-guided core biopsies, but in one review of core biopsies in general that included nearly 29,000 lesions, the incidence of serious complications was less than 1%. 19 This study reported an incidence of hematoma requiring treatment of 0.09% and infections requiring antibiotics in 0.15%. The biopsies in these studies were performed with 11- and 14-gauge needles, whereas MRI-guided biopsies are often done with 9-gauge needles, and it is possible that the incidence of significant bleeding with MRI-guided biopsies may be higher than with smaller gauge needles, though still relatively low. Perlet et al, in a multicenter study, had serious bleeding in 3 out of 334 (0.9%) biopsies that required surgical intervention and 1 case of infection requiring antibiotics. 2 It is common practice to stop anticoagulation, particularly with warfarin or chronic low-dose aspirin prior to the procedure. However, there is some evidence that MRI-guided biopsies can be safely performed even in women who are anticoagulated. In a very small series of core biopsies of 18 lesions in women on anticoagulation with warfarin (11 lesions), aspirin (6 lesions), or heparin (1 lesion), no clinically important complications occurred. 20 Similarly, Somerville et al reported on a series of core biopsies done on 200 women on aspirin (180), warfarin (16), and other nonsteroidal anti-inflammatory drugs (NSAIDs; 4). 21 They compared these women to 855 others who were not on anticoagulation and found that there was a statistically significant incidence of bruising among the anticoagulated group (34 vs. 26.5%, p = 0.035), but there was no significant difference in the occurrence of hematomas. Therefore, both of these studies concluded that core biopsies can be done on women on anticoagulation especially if stopping the anticoagulant is associated with more risk than the risk of hematoma formation. However, if there is no compelling reason to continue anticoagulation, both of the authors continue to stop medication before the procedure. If warfarin cannot safely be stopped, it is advisable to check the international normalized ratio (INR) prior to the biopsy to be certain it is not above therapeutic range. A potential complication unique to MRI-guided procedures is reaction to gadolinium. All women having MRI-guided biopsies have already had an MRI examination with gadolinium, presumably without untoward effect, but there is no guarantee that a reaction will not occur. However, serious reactions to gadolinium-based contrast are uncommon. In one series of over 158,000 examinations in which gadolinium was administered intravenously, only 15 cases required treatment, with the most common reaction being hives. 22 The presence of an implant is not an absolute contraindication to MRI-guided biopsy although it can make the procedure technically challenging. When there is an implant present, mention of the remote possibility of inadvertent implant rupture should be included in the consent process. In obtaining consent, it is advisable to include a discussion of clip placement and the need for a postprocedure mammogram to document the location of the clip. Some women object to the presence of a clip, but when presented with the reasons a clip is needed, the advantages of clip placement and the lack of documented complications associated with clips, patients usually agree to have one placed. It should be explained that a clip is needed in case more tissue needs to be removed should the biopsy results come back showing malignancy or a high-risk lesion and without the clip, localization will need to be done with MR guidance rather than with mammographic guidance, which is faster, easier, and more comfortable for the patient. In addition, if the biopsy results are benign and concordant, the clip indicates the site of biopsy on future MR examinations. Because AB-MR is done for screening, it is likely that these patients will have repeated MRIs and having documentation of the location of the biopsy may be helpful in future interpretation. The fact that the patient will not experience pain or any other untoward effects from the clip, that it will not set off metal detectors, and that it will not interfere with future MRI examinations can be stressed. It is quite helpful to have an actual clip available to show patients who are concerned to show how tiny they are. It is good practice to tell the patient when the pathology results are likely to be available and to formulate a plan for conveying the results. Ideally, the results should be given to the patient by the radiologist who performs the procedure as he/she is the person who can best assess concordance of imaging and pathology. A management recommendation should always be included in the report of the procedure and ideally, this recommendation should be conveyed directly to the patient. The steps in performing MRI-guided core biopsies are listed here with details of each step outlined in this section. Position patient. Do pre- and postcontrast T1-weighted sequences. This can be in the axial or sagittal plane, depending on your equipment. Target the lesion. Cleanse the skin and apply local anesthetic. Position the sampling sheath using the introducer. Replace the metal introducer with the plastic obturator and scan to be sure position of the sheath is correct. Obtain samples. Scan with obturator in place to be sure the lesion was successfully sampled. Place a clip. Scan to confirm clip placement (optional). Do mediolateral (ML) and craniocaudal (CC) mammograms to confirm clip location. It is important that the patient’s breast be positioned in the breast coil by an experienced technologist. Not uncommonly, technologists who are trained in performing MRI examinations are not accustomed to positioning the breast. When starting an MR biopsy program, if the MRI technologists are not familiar with the principles of positioning, technologists from the breast imaging section can assist initially in positioning the patient for MRI biopsies until the MRI technologists are comfortable and proficient in performing this task. Positioning for MRI biopsies is similar to positioning for stereotactic breast biopsies. Medial and lateral breast tissue should be pulled into the coil as much as possible and the nipple should be in profile and be in the center of the coil. Having a well-trained technologist position the patient can make the difference between allowing access to a lesion in a difficult position in the breast and having to cancel the procedure. Time and effort should also be given to making the patient as comfortable as possible before starting in order to minimize the possibility of movement during the biopsy. If an implant is present, implant displacement technique should be utilized to move the implant out of the compression device as much as possible, just as is done with stereotactic biopsy positioning ( ▶ Fig. 8.4). This may not be possible, however, depending on the location of the implant and the degree of capsule formation. If the implant cannot be safely displaced away from the lesion, core biopsy may not be possible. In these cases, MRI-guided needle localization can be attempted. The compression grid or pillar and post should be placed at the surface of the breast closest to the lesion, either lateral or medial. Some devices allow a grid to be placed simultaneously on both the lateral and medial surfaces and this can be advantageous if the lesion is close to the midline. Under compression, the lesion may move closer to one side of the breast or the other and having the ability to approach from either the medial or the lateral surface can minimize the amount of tissue that needs to be traversed in order to reach the target. Compression should be firm enough to prevent lesion movement but not overly tight so as to compromise blood flow and potentially prevent adequate lesion visualization. In a study of the effect of breast compression evaluating the degree of compression applied during biopsy procedures and comparing lesion size in the noncompressed to the compressed breast, Khouli et al showed that the degree of compression applied during the biopsy procedure varied considerably and that the application of compression resulted in decreased accuracy in lesion size determination and visibility. 23 In most cases, intravenous contrast will need to be administered not only to locate the lesion, but also to be certain the lesion is still enhancing. It is advantageous to obtain a precontrast sequence to be certain positioning is correct, fat suppression is adequate, and the fiducial marker can be seen. In addition, having a precontrast sequence allows for subtraction to be performed if necessary for optimal visualization of the abnormality. Power injection is not absolutely necessary because analysis of kinetics is not performed. However, it is important to be able to inject without the need to move the patient out of the bore of the magnet in order to minimize the possibility of patient movement. Fig. 8.4 Postcontrast T1-weighted image shows nonmass enhancement in a woman with an implant (a). Magnetic resonance imaging guided biopsy was recommended. The patient was positioned in the biopsy coil with the implant displaced, allowing for successful sampling (b). All of the commercially available computer-aided detection (CAD) systems have automated targeting software that simplifies identifying the location of a lesion in the breast. However, just as pilots need to know how to fly airplanes manually despite the availability of autopilot, it is important for radiologists who do MRI-guided biopsies to know how to target without the aid of the software programs in case of computer or software malfunction. For targeting using the fenestrated grid, a fiducial marker must be placed and included on the images. With the automated software programs, fiducials are either built into the compression device or placed at a preset location within the fenestrated compression grid. For the automated systems, scanning can be performed in either the sagittal or the axial plane. The lesion is marked and the software determines the appropriate opening in the fenestrated grid, the appropriate opening in the needle guide, and the depth of the lesion from the skin surface ( ▶ Fig. 8.5). For manual targeting with the fenestrated grid, commercially available markers that are visible on T1-weighted images can be used, although a simple vitamin E capsule will suffice. For manual targeting, the marker is placed in any one of the grid openings. Scanning is done either in the sagittal plane or in the axial plane with reconstruction. On the MRI workstation, the lesion is marked on the sagittal images and the slices are scrolled back to the grid to determine the appropriate opening to use. This represents the x– and y-axis location of the lesion. For the depth (z-axis) location, the number of slices between the skin surface and the lesion is determined and then multiplied by the slice thickness to get the depth. For example, if the skin surface is seen on image 3 and the lesion on image 13, there are 10 slices between the two. If the slice thickness is 3 mm, the lesion is 10 × 3 or 30 mm from the skin. These steps are illustrated in ▶ Fig. 8.6. The orientation of the patient on the workstation is different from the orientation of the patient in the magnet. In order to ensure that the correct grid opening is used, all of the compression grid device manufacturers have printed sheets that allow indication of which opening should be targeted ( ▶ Fig. 8.7). This is then brought into the magnet room and flipped to reflect the patient’s orientation. For the pillar-and-post device, either the axial or the sagittal plane can be used. With the patient in compression, the pillar is set to zero location in both the x– and y-axes. A fiducial marker is placed and imaging is performed. The fiducial is marked on the slice on which it appears and a reference line is placed on the workstation. The x-axis (horizontal) location is determined by the number of slices between the fiducial and the lesion multiplied by the slice thickness. The vertical location (y-axis) is calculated as the distance from the lesion to the fiducial reference line. Once the x– and y-axis locations are determined, the fiducial is removed, and the patient rescanned to be certain that the location of the pillar is correct. The z-position is measured as the distance from the skin surface to the lesion to determine the depth. These steps are illustrated in ▶ Fig. 8.8. Fig. 8.5 Targeting with computerized targeting software. The lesion is found on either axial or sagittal sequence. A cursor is placed on the lesion and the appropriate grid opening, opening in the needle guide, and depth are indicated.
8.2 Targeted Ultrasound
8.3 Before the Biopsy
8.3.1 Equipment
8.3.2 Patient Preparation and Consent
8.3.3 Technique
8.3.4 Patient Positioning
8.3.5 Targeting
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree