Evaluation of neck lesions in the pediatric population can be a diagnostic challenge, for which magnetic resonance (MR) imaging is extremely valuable. This article provides an overview of the value and utility of MR imaging in the evaluation of pediatric neck lesions, addressing what the referring clinician requires from the radiologist. Concise descriptions and illustrations of MR imaging findings of commonly encountered pathologic entities in the pediatric neck, including abnormalities of the branchial apparatus, thyroglossal duct anomalies, and neoplastic processes, are given. An approach to establishing a differential diagnosis is provided, and critical points of information are summarized.
- •
Magnetic resonance imaging does not involve ionizing radiation, and is ideally suited for characterization of pediatric neck lesions located in anatomically complex areas such as the floor of the mouth and deep neck spaces.
- •
The leading differential considerations for a cystic lesion on the floor of the mouth include a thyroglossal duct cyst, a dermoid/congenital inclusion cyst, a ranula, and a foregut duplication cyst.
- •
Anomalies of the second branchial cleft are the most common of the branchial-apparatus anomalies. These lesions typically displace the submandibular gland anteriorly and the sternocleidomastoid muscle posterolaterally.
- •
A nontender fusiform mass involving unilateral sternocleidomastoid muscle in a young infant is often pathognomonic of fibromatosis colli.
- •
Lymphatic malformations are large transspatial, multiloculated masses with heterogeneous signal intensity that may demonstrate fluid levels secondary to hemorrhage.
- •
An enhancing solid mass, associated with the carotid sheath and unilateral Horner syndrome, should raise concern for neuroblastoma.
- •
Aggressive soft-tissue masses in the pediatric head and neck with transspatial involvement, especially when accompanied by osseous destruction, are most commonly associated with rhabdomyosarcomas.
Introduction
Evaluation of neck lesions in the pediatric age group can be a diagnostic challenge. The evaluation begins with clinical history and thorough physical examination. Imaging, either ultrasonography (US), computed tomography (CT), or magnetic resonance (MR) imaging, is used to further characterize these lesions. US is a quick imaging modality, which does not involve ionizing radiation and is helpful in characterizing or in confirming cystic nature of lesion and, to a certain extent, in defining the relationship of lesions with surrounding normal structures. However, US is limited in evaluating lesions that are deep seated or very small. CT is superior to US regarding cross-sectional evaluation of masses that are deep seated. CT is also superior for detection of calcification, and with the new ultrafast scanners CT is the investigation of choice in the setting of an emergency involving trauma, acute infection, or airway compromise. However, CT involves ionizing radiation, which can be potentially harmful in the pediatric age group to sensitive organs such as the thyroid gland. MR imaging does not involve ionizing radiation, has superior contrast resolution, and has an inherently high signal-to-noise ratio, making it ideal for evaluating various neck lesions. MR is ideally suited for evaluation of masses located in anatomically complex areas such as the floor of the mouth and deep neck spaces. MR can characterize a lesion better, can accurately demonstrate the full extent of the mass along with its relationship with the surrounding structures, and provides important information for presurgical planning.
This article provides an overview of the utility of MR imaging in the evaluation of various neck lesions in the pediatric population.
Technical aspects
A typical neck MR study includes multiplanar T1-weighted and T2-weighted images. It is recommended that T2-weighted images be done with fat saturation. Depending on the clinical indication for the study, the slice thickness needs to be tailored according to the lesion being evaluated. For example, for evaluation of subcentimeter lesions and small tracts and fistulae, the slice thickness may have to be as thin as 2 mm. Addition of HASTE (Half-Fourier Single short Turbo Echo) sequences is particularly valuable for identifying cystic lesions and fluid-filled sinus tracts, shown in Figs. 1 and 2 . Contrast-enhanced MR imaging is essential for the evaluation of most lesions that are congenital, inflammatory/infectious, or of neoplastic etiology. Postcontrast T1 imaging with fat suppression is helpful in correctly identifying subtle enhancement. Addition of diffusion-weighted (DW) imaging is particularly useful in the evaluation of suspected dermal inclusion cysts (see Fig. 1 ), abscesses, or neoplastic lesions, for better characterization of these lesions and help in arriving at a specific diagnosis. At their institution the authors routinely use DW imaging and obtain apparent diffusion coefficient (ADC) maps in MR evaluation of the neck. The average duration of a neck MR imaging study is approximately 30 to 40 minutes. The older children are able to hold still for the duration of the MR study without the use of sedation. However, infants and young children often require preprocedural sedation to maintain a motionless state, thus ensuring high-quality MR imaging.


A discussion of the detailed anatomy of the neck spaces is beyond the scope of this article. The reader is referred to further excellent literature for this information. The distribution of the different neck lesions according to their location is summarized in Table 1 .
| Location | Lesions |
|---|---|
| Floor of the mouth | Thyroglossal duct cyst (midline) Foregut duplication cyst Dermoid, epidermoid Ranula Lymphatic malformation Teratoma Vascular lesions (ie, hemangioma, venous vascular lesions) |
| Periauricular | Lymphadenitis Anomalies of first branchial cleft Atypical mycobacterial infection Lymphatic malformation |
| Submandibular | Lymphadenitis Anomalies of second branchial cleft Atypical mycobacterial infection Lymphatic malformation Vascular lesions (ie, hemangioma, venous vascular lesions) |
| Carotid | Lymphadenitis Branchial-cleft anomalies Lymphatic malformation Neurogenic tumors: neuroblastoma, neurofibroma Vascular lesions (ie, hemangioma, venous vascular lesions) |
| Neck anterior | Thyroglossal duct cyst (midline or within 2 cm of midline) Thymic cyst/ectopic thymus Lymphatic malformation Lymphadenitis Teratoma |
| Supraclavicular | Lymphoma Metastatic disease Lymphadenitis Lymphatic malformation |
| Posterior neck occipital | Lymphadenitis Lymphoma Lymphatic malformation Vascular lesions (ie, hemangioma, venous vascular lesions) |
Knowledge of the embryologic origin of neck structures is helpful in the understanding and detection of pediatric neck lesions. Relevant embryology is discussed here, along with thyroglossal duct cysts (TGDCs), anomalies of the branchial apparatus, and thymic cysts.
Technical aspects
A typical neck MR study includes multiplanar T1-weighted and T2-weighted images. It is recommended that T2-weighted images be done with fat saturation. Depending on the clinical indication for the study, the slice thickness needs to be tailored according to the lesion being evaluated. For example, for evaluation of subcentimeter lesions and small tracts and fistulae, the slice thickness may have to be as thin as 2 mm. Addition of HASTE (Half-Fourier Single short Turbo Echo) sequences is particularly valuable for identifying cystic lesions and fluid-filled sinus tracts, shown in Figs. 1 and 2 . Contrast-enhanced MR imaging is essential for the evaluation of most lesions that are congenital, inflammatory/infectious, or of neoplastic etiology. Postcontrast T1 imaging with fat suppression is helpful in correctly identifying subtle enhancement. Addition of diffusion-weighted (DW) imaging is particularly useful in the evaluation of suspected dermal inclusion cysts (see Fig. 1 ), abscesses, or neoplastic lesions, for better characterization of these lesions and help in arriving at a specific diagnosis. At their institution the authors routinely use DW imaging and obtain apparent diffusion coefficient (ADC) maps in MR evaluation of the neck. The average duration of a neck MR imaging study is approximately 30 to 40 minutes. The older children are able to hold still for the duration of the MR study without the use of sedation. However, infants and young children often require preprocedural sedation to maintain a motionless state, thus ensuring high-quality MR imaging.
A discussion of the detailed anatomy of the neck spaces is beyond the scope of this article. The reader is referred to further excellent literature for this information. The distribution of the different neck lesions according to their location is summarized in Table 1 .
| Location | Lesions |
|---|---|
| Floor of the mouth | Thyroglossal duct cyst (midline) Foregut duplication cyst Dermoid, epidermoid Ranula Lymphatic malformation Teratoma Vascular lesions (ie, hemangioma, venous vascular lesions) |
| Periauricular | Lymphadenitis Anomalies of first branchial cleft Atypical mycobacterial infection Lymphatic malformation |
| Submandibular | Lymphadenitis Anomalies of second branchial cleft Atypical mycobacterial infection Lymphatic malformation Vascular lesions (ie, hemangioma, venous vascular lesions) |
| Carotid | Lymphadenitis Branchial-cleft anomalies Lymphatic malformation Neurogenic tumors: neuroblastoma, neurofibroma Vascular lesions (ie, hemangioma, venous vascular lesions) |
| Neck anterior | Thyroglossal duct cyst (midline or within 2 cm of midline) Thymic cyst/ectopic thymus Lymphatic malformation Lymphadenitis Teratoma |
| Supraclavicular | Lymphoma Metastatic disease Lymphadenitis Lymphatic malformation |
| Posterior neck occipital | Lymphadenitis Lymphoma Lymphatic malformation Vascular lesions (ie, hemangioma, venous vascular lesions) |
Knowledge of the embryologic origin of neck structures is helpful in the understanding and detection of pediatric neck lesions. Relevant embryology is discussed here, along with thyroglossal duct cysts (TGDCs), anomalies of the branchial apparatus, and thymic cysts.
Thyroglossal duct cyst
During the fourth week of gestation, the thyroid primordium originates as a diverticulum from the floor of the pharynx (tuberculum impar) at the site of the future foramen cecum at the base of the tongue. The gland migrates inferiorly through the peripharyngeal connective tissue just anterior to the hyoid bone and laryngeal cartilages and descends in the neck anterior to the thyrohyoid membrane and deep to the strap muscles, reaching its final position in the lower midline of the neck by the seventh week of gestation. The anlage of the gland is connected to the tongue by the thyroglossal duct during its migration. The thyroglossal duct obliterates between the fifth and tenth week of gestation, leaving behind a proximal remnant at the foramen cecum and a distal remnant: the pyramidal lobe of the thyroid gland. The duct is intimately associated with the developing hyoid bone. Failure of obliteration of the thyroglossal duct before the formation of the mesodermal anlage of the hyoid bone results in its persistence during development and after birth. The line defined by the embryologic descent of the thyroid gland is the site where the anomalies of the thyroglossal duct can occur.
Anomalies of the thyroglossal duct constitute the majority of the congenital midline cervical masses seen in children. It has been estimated that they account for about 70% of congenital neck anomalies, and are the second most common benign neck mass in pediatric patients behind benign cervical lymphadenopathy. TGDCs are approximately 3 times more common than anomalies of the branchial apparatus.
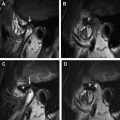
TGDCs arise as cystic expansion of the remnant of the thyroglossal duct tract. TGDCs are located adjacent to the hyoid bone (60%), between the hyoid bone and the base of the tongue (24%), and infrahyoid (13%). Three percent of TGDCs may be intralingual in location. TGDCs are intimately related to the hyoid bone, either juxtahyoid or infrahyoid. Most of these (75%) are in or slightly off the midline (25%) but within 2 cm of the midline ( Fig. 3 A). The paramedian location is usually seen with the infrahyoid TGDCs ( Fig. 3 B).
On MR imaging, a noncomplicated TGDC is hypointense on T1, hyperintense on T2, and bright on HASTE sequences, reflecting fluid content. Sagittal MR images are particularly useful in determining the extent of the lesion before resection. Such lesions may be unilocular or multiloculated and occasionally have septations. There is no peripheral rim enhancement unless there has been prior inflammation. This classic appearance is seen in fewer than half of cases. A peripheral rim of enhancement is seen if there has been prior infection or inflammation ( Fig. 4 ). In the case of infection or hemorrhage a thick irregular rim may be demonstrated, and the signal intensity of the contents may vary according to the presence of proteinaceous or hemorrhagic debris within the cyst. Thickening of the surrounding strap muscles and cutaneous induration of the subcutaneous fat can be related to TGDC inflammation. Association of a solid mass with a TGDC usually represents ectopic thyroid tissue. Coexisting malignancy may be found within 1% of TGDCs. Malignancy usually arises from ectopic thyroid tissue and is typically a papillary carcinoma. Papillary carcinoma may be seen on MR imaging as a soft-tissue mass, which enhances with contrast and usually contains foci of mineralization/calcification.
Thyroglossal duct cysts are treated by surgical excision. Typically a Sistrunk procedure is used, which involves complete resection of the entire TGDC along with excision of the midportion of the hyoid bone and any associated tract. Occasionally, following simple cyst excision or even the Sistrunk procedure, the TGDC may recur. MR imaging is again valuable in evaluation of recurrent cysts. Fig. 5 illustrates a case of recurrence of a TGDC following the Sistrunk procedure.
What the Ear/Nose/Throat (ENT) Surgeon Wants to Know
- 1.
Whether the TGDC is a single cyst or if it is multifocal; location with regard to the surrounding anatomy: foramen cecum, base of tongue musculature, and hyoid bone.
- 2.
Whether the thyroid gland is present in a normal location in the lower neck. Is there evidence of lingual thyroid?
- 3.
If there is a recurrence the location and size of the recurrent TGDC, and if there are more than one foci of recurrence.
Lingual thyroid
Arrest of the migration of the thyroid anlage between 3 and 7 weeks of gestation results in an ectopic location of the thyroid gland at the tongue base, usually at foramen cecum. The imaging features of the lingual thyroid resemble that of thyroid gland tissue, that is, iso- to hyperintense on T1-weighted images and mild to strikingly hyperintense on T2-weighted images, with more enhancement than for the tongue ( Fig. 6 ). Once a lingual thyroid is identified, it is important to assess by imaging whether an orthotopic thyroid tissue is present and if there are additional sites of ectopic thyroid tissue in the neck. A radioisotope scan is usually performed to help assess or confirm such findings.
Congenital inclusion cysts/dermoids and epidermoids
The term congenital inclusion cyst is commonly used in reference to both dermoid and epidermoid cysts. These cysts arise from elements of the ectoderm and are lined by squamous epithelium. Dermoid cysts include components of all 3 layers of the ectoderm and may include adnexal structures such as teeth, hair, and sebaceous glands. Dermoid cysts may also contain lipid material derived from sebaceous secretions. Epidermoid cysts include debris from the desquamated epithelium from 2 layers of the ectoderm containing keratin and cholesterol derived from the breakdown of cell membranes.
The oral cavity is the second most common location of dermoid cysts in the head and neck regions, the most common location being the orbital area.
Dermoid cysts in the oral cavity are in the region of the floor of the mouth and are typically found in the midline. These cysts may be located on the dorsum of the tongue and the hard or soft palate. Sublingual dermoid cysts are centered above the mylohyoid muscle and present as submucosal lesions in the floor of the mouth. Submental dermoid cysts are centered below the mylohyoid muscle.
On MR imaging dermoid cysts are usually well-demarcated, unilocular midline masses located within the submandibular or the sublingual space. Presence of fat within the lesion is a characteristic feature of a dermoid cyst. Dermoid cysts are most commonly moderately hyperintense on T1-weighted images because of the presence of lipids; however, the signal characteristics on T1 images may be variable and can be isointense relative to muscle. On T2-weighted images they are usually hyperintense. Heterogeneity, fluid-fluid levels, and calcification may be present ( Fig. 7 ). Many dermoid cysts show restricted diffusion.
Epidermal inclusion cysts have fluid-signal characteristics that are T1-hypointense and T2-hyperintense. Diffusion restriction is a characteristic feature of epidermal inclusion cysts or epidermoids (see Fig. 1 ).
ENT Surgeon Comment
MR imaging is valuable in demonstrating the exact location of the dermoid cyst with respect to the mylohyoid muscle using the multiplanar imaging capability, which is most helpful for planning surgical resection and approach. Dermoid cysts located superior to the mylohyoid muscle typically require a transoral surgical approach, whereas those cysts located inferior or superficial to the mylohyoid muscle require an external submandibular approach.
Ranula
A ranula is a pseudocyst containing extravasated mucus from the sublingual salivary glands. Ranulas are considered pseudocysts, as they lack a true epithelial lining. A simple ranula is a cystic collection confined to the sublingual space in the floor of the mouth. A diving or plunging ranula typically presents with a swelling in the submandibular region or upper neck as the result of mucus extravasating inferior to the mylohyoid muscle.
On MR imaging, a ranula typically demonstrates signal consistent with fluid that is hypointense on T1-weighted images and hyperintense on T2-weighted images ( Fig. 8 ). Thin linear enhancement of the wall may be seen following contrast. If there has been recent infection or inflammation, the wall may be thick with irregular enhancement. A simple ranula has either an oval or lenticular shape, and if bilateral may assume a horseshoe shape. On imaging a plunging ranula has a comet shape and may have a tail in the sublingual space ( Fig. 9 ). The presence of the tail is a characteristic feature of a plunging ranula, and when they are large enough they may extend into the upper neck and parapharyngeal space.
ENT Surgeon Comment
Management of a ranula typically requires resection of the ipsilateral sublingual gland and intraoral drainage of the mucus collection. Simple transoral drainage and/or marsupialization of a ranula may be performed, but has a high recurrence rate. Therefore, transoral resection of the sublingual gland with drainage of the mucus collection is a recommended technique. The submandibular duct and lingual nerve should be carefully preserved, as they typically run along the medial aspect of the dissection. Complete resection of the pseudocyst is unnecessary, as there is no epithelial lining of the collection and the origin of the fluid is the sublingual gland, which is completely excised. Confirmation of the diagnosis of a ranula can be made by analyzing the fluid composition, as it typically will have lower sodium, chloride, and glucose levels, and higher amylase levels, than a similar-appearing collection from a cystic hygroma. A biopsy of the wall of the pseudocyst may also be sent for confirmation.
Anomalies of the branchial apparatus
Anomalies of the branchial apparatus account for 17% of all pediatric neck masses, and are the second most common developmental neck abnormality after the TGDC.
To understand the different anomalies arising from the branchial apparatus, it is crucial to have knowledge about their embryologic origin. All the structures of the neck are derived from the branchial apparatus, which consists of paired pharyngeal arches, clefts, pouches, and membranes. During the fourth to seventh weeks of the human embryonic development 6 paired branchial arches are initially formed. The first branchial arch is either not formed or quickly degenerates in the body. Five ectodermal-lined branchial clefts and 5 endodermal-lined pharyngeal pouches separate the 6 arches. A closing membrane is present at the interface of the pharyngeal pouches and the branchial clefts. The external auditory canal forms from the first cleft, and the tympanic cavity, mastoid air cells, and Eustachian tube develop from the first pouch. The palatine tonsil is a derivative of the second pouch. The second cleft disappears completely. Incomplete obliteration of the branchial apparatus results in the formation of branchial cysts, sinuses, or fistulae. Regardless of the different theories about the origin of these congenital cervical anomalies, the most popular accepted hypothesis concerns the branchial remnants that lead to anomalies of the branchial apparatus, of which anomalies of the second branchial cleft are the most common. With continued development of the neck, the second branchial arch overgrows the third and the fourth branchial clefts and fuses with the lateral branchial wall. An ectodermal-lined cavity known as the cervical sinus of His is formed. Incomplete obliteration of the cervical sinus plays an important role in the development of anomalies of the second branchial cleft. A line drawn from the oropharyngeal tonsillar fossa to the supraclavicular region of the neck represents the site of anomalies of the second branchial cleft.
Based on their location, anomalies of the branchial apparatus are classified according to the proposed pouch or cleft of origin. The majority are anomalies of the second branchial apparatus, followed by those of the first branchial cleft. Anomalies of the third and particularly the fourth branchial apparatus are extremely rare. Table 2 summarizes the location and common clinical presentation of anomalies of the branchial apparatus.
| Type | Location | Clinical Presentation |
|---|---|---|
| First | External auditory canal, parotid region | Mass around external auditory canal, chronic unexplained otorrhea, or recurrent parotid gland abscess |
| Second | Submandibular region, lateral to carotid vessels | Painless mass at mandibular angle or lateral neck in a child or young adult |
| Third | Posterior triangle of neck, usually left side | Unexplained recurrent deep or lateral neck infection/abscesses Draining cervical sinus Mass in posterolateral neck expanding after respiratory tract infection |
| Fourth | Sinus tract opening at pyriform sinus usually on the left | Mass on lower third of neck usually on the left side Recurrent neck abscesses Recurrent suppurative thyroiditis |
MR imaging is particularly useful in the evaluation of anomalies of the branchial apparatus and in understanding their relationship to the surrounding structures.
Anomalies of the First Branchial Apparatus
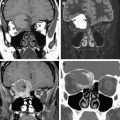
Anomalies of the first branchial cleft can occur anywhere along the embryologic tract extending from the submandibular triangle and terminating at the cartilaginous-bony junction of the external auditory canal, coursing through the parotid gland, with a variable relationship with the facial nerve. The majority of first-branchial anomalies are unilocular cysts, within the parotid gland or around the external auditory canal. Fistulae typically extend from the floor of the external auditory canal through the parotid gland, to an opening located between the sternocleidomastoid muscle, hyoid bone, and angle of the mandible. The appearance of the cyst on MR imaging depends on the contents of the cyst. Simple cystic lesions demonstrate fluid-signal characteristics with hypointense signal on T1-weighted images and hyperintense signal on T2-weighted images ( Fig. 10 ). With increasing protein content or infections there is a higher signal on T1-weighted images. There may be enhancement and a thick wall if the cyst has been infected. A first-branchial anomaly has a variable relation to the facial nerve. The location of the branchial anomaly and its relationship to the facial nerve should be specifically determined before surgery if possible. MR imaging is especially useful in determining these important factors prior to surgery. Care must be taken during surgery, as the nerve may be displaced laterally if the anomaly runs medial to the nerve, putting the nerve at risk during the approach to the anomaly.
Anomalies of the Second Branchial Cleft
The typical location of these lesions is in the submandibular space along the anterior border of the sternocleidomastoid muscle, but can be anywhere along the developmental path of the second branchial arch.
On MR imaging these lesions typically demonstrate a cystic nature, with low signal on T1-weighted images and high signal on T2-weighted images. Signal characteristics may vary according to the protein content within the cyst. With high proteinaceous fluid content there may be increased signal on T1, and decreased or intermediate T2 signal intensity. The most common of the cysts of the second branchial cleft is the Bailey type 2. Such cysts displace the submandibular gland anteriorly, push the carotid space vessels medially or posteromedially, and displace the sternocleidomastoid muscle posterolaterally ( Fig. 11 ). The typical path of an anomaly of the second branchial cleft is over the glossopharyngeal nerve and hypoglossal nerves, between the internal and external carotid arteries, penetrating through the pharyngeal musculature to communicate with the oropharynx in the region of the tonsillar fossa. Occasionally a beak sign may be seen as a small soft tissue pointing medially between the internal and external carotid arteries, and is considered a pathognomonic imaging feature of a cyst of the second branchial cleft.
Anomalies of the Third and Fourth Branchial Clefts
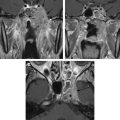
Developmental anomalies of the third and the fourth branchial clefts are extremely rare, and are almost always formed on the left side. Cysts of the third branchial cleft usually present as a mass in the posterior cervical triangle, and are considered the most common congenital lesion of the posterior cervical space after lymphangiomas. A characteristic fistula of third-branchial origin communicates externally along the anterior border of the sternocleidomastoid muscle, similar to a second-branchial anomaly, only more caudally. A third-branchial anomaly follows the predicted embryologic course extending from the supraclavicular fossa to the lateral aspect of the piriform sinus. The tract descends within the carotid sheath posterior to the common and internal carotid arteries and anterior to the vagus nerve, between the hypoglossal and glossopharyngeal nerves, and penetrates the thyrohyoid membrane to communicate with the upper part of the piriform sinus, anterior to the superior laryngeal nerve. The possibility of a cyst of the third branchial cleft can be suggested on MR imaging when the lesion is situated in the lateral compartment of the neck, posterior to the common carotid artery and jugular vein, and is in the posterior triangle of the neck. These lesions may extend inferiorly to the level of the thyroid gland.
Differentiating anomalies of the third branchial cleft from those of the fourth may be difficult because both have a relationship with the piriform sinus. The difference between these two lesions is in their relationship to the superior laryngeal nerve (nerve of the fourth arch). The lesions above this structure originate from the third branchial cleft and those below are derived from the fourth branchial cleft. The fourth-branchial anomaly typically opens into the apex of the piriform sinus.
Cysts of the third branchial cleft are mostly seen as a unilocular cystic mass on MR imaging. The signal depends on the contents and the wall thickness, and enhancement and surrounding changes vary according to the associated inflammatory changes. An internal fistula arising from the apex of the piriform sinus would be a remnant of the fourth pouch. When the sinus tracts are fluid-filled or inflamed they may be seen on MR imaging with the help of HASTE sequences and fat-suppressed, contrast-enhanced T1-weighted sequences. However, the gold standard for evaluation of these sinuses and fistulae is direct visualization by fluoroscopy following injection of contrast material ( Figs. 12 and 13 ).