Although uncommon, sinonasal malignancies and aggressive inflammatory processes are entities every radiologist will encounter during the evaluation of routine sinus imaging studies. A high index of suspicion is necessary for prompt diagnosis. It is important to consider aggressive inflammatory disease in all patients having routine sinus computed tomography because any delay in diagnosis can adversely affect the patients’ care. Magnetic resonance (MR) will often provide a better assessment of the lesion extent, allowing for better surgical treatment. MR is crucial for the accurate assessment of neoplastic lesions. A proficient understanding of the complex anatomy of the region is essential.
- •
A thorough knowledge of the anatomy of the sinonasal region and adjacent structures is essential for proper evaluation.
- •
Magnetic resonance (MR) imaging is a vital imaging modality for the assessment of aggressive and neoplastic diseases affecting the nasal cavity and paranasal sinuses.
- •
MR imaging is much superior to computed tomography at separating tumor from inflammatory mucosal disease, depicting the full extent of disease involvement, and evaluating perineural tumor spread.
Introduction
Most radiology practices receive daily requests to perform diagnostic imaging studies in patients with sinus symptoms. Most problems that require evaluation will be caused by common inflammatory diseases that require little or no acute treatment. Importantly, however, the patients’ symptoms may occasionally have other causes, such as a more aggressive inflammatory process or a malignant neoplasm. There is often a significant overlap in the presenting symptoms of benign inflammatory, aggressive inflammatory, and neoplastic paranasal sinus diseases. Some patients with underlying neoplasms may even be mistakenly treated with one or more courses of antibiotics before imaging is obtained and other possibilities are considered. In an immunocompromised host, more aggressive causes must be considered as soon as possible because any delay in diagnosis may lead to significant morbidity or even mortality.
Many patients with neoplasms present with advanced disease at diagnosis because of the failure to consider this possibility until late in the course. Additionally, given the capacious nature of the sinuses, tumors can grow large before becoming symptomatic. Sinonasal neoplasms tend to present at an advanced stage and have a poor overall prognosis. Clinically, they have nonspecific presenting signs, including nasal stuffiness, bleeding, pain, and numbness. Pain and numbness may herald the onset of perineural tumor spread by the lesion. Cancers of the head and neck account for approximately 4% of all malignancies. Sinonasal tumors make up 3% to 4% of head and neck cancers. There are several tissue types that are normally found in the sinonasal cavity, including epithelial (neuroendocrine, schneiderian, squamous, olfactory), mesenchymal (bone, cartilage, fibrous), muscle, nervous, and vascular. Because of this, there is a wide spectrum of neoplasms that may originate in the sinonasal cavity. Most sinus cancers are squamous cell carcinoma (80%) and these most commonly involve the maxillary sinus (36%–80%) followed by the nasal cavity (25%–44%) and ethmoid sinuses (10%). The sphenoid and frontal sinuses are rare sites of origin.
The purpose of this article is to explore the differentiating imaging features of aggressive inflammatory versus neoplastic lesions of the paranasal sinuses. A secondary focus is to look at common patterns of spread of aggressive sinus lesions to adjacent structures.
Imaging
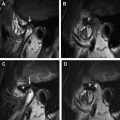
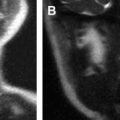
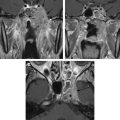
Computed tomography (CT) and magnetic resonance (MR) imaging can be considered complimentary diagnostic studies for the evaluation of aggressive inflammatory and neoplastic diseases of the sinuses ( Figs. 1 and 2 ). CT is typically the first diagnostic test used for the evaluation of the paranasal sinuses. With thin slices and multiplanar reformations, CT does a satisfactory job at evaluating most disease processes involving the sinuses. CT is excellent at evaluating the bony anatomy, orbital fat, bony cortex, and for depicting the internal architecture of some fibro-osseous (see Fig. 1 ) and other bony lesions (see Fig. 2 ). MR imaging is a better choice for identifying the full extent of aggressive sinonasal disease that spread into the orbit, skull base, brain, and cavernous sinus. It is more definitive than CT for separating tumor from inflammatory mucosal disease and retained secretions. MR imaging is superior to CT for detecting the full extent of perineural spread of tumors. In many cases, CT and MR imaging play complimentary roles. Important things to evaluate include bone remodeling versus destruction, presence of calcification, inherent T1 and T2 signal intensity, lesion cellularity ( Fig. 3 ), degree of contrast enhancement, vascularity, and the involvement of the orbital, premaxillary, and retromaxillary fat. Detecting spread into the orbit, skull base, cavernous sinus, and brain as well as the presence of perineural tumor spread will usually alter the type of treatment. The accurate assessment of tumor spread will also influence the discussion of viable treatment options and overall disease prognosis with patients. Cerebral angiography or MR angiography may be helpful in the management of vascular sinonasal masses (see Fig. 2 ; Fig. 4 ). The radiologist’s job is to separate nonaggressive inflammatory sinus disease from more aggressive inflammatory or neoplastic processes. Patients can then undergo a biopsy for the final diagnosis and appropriate treatment.

A recommended CT protocol includes the acquisition of thin (0.5–1.5 mm) axial images that are reconstructed in both soft tissue and bone algorithms (see Fig. 2 ). These images are then used to generate thin (1.25–2.5 mm), 2-dimensional reconstructions in the coronal and sagittal planes (see Fig. 1 ). A typical MR imaging protocol includes axial, coronal, and sagittal noncontrast T1-weighed images, axial diffusion-weighted images, and axial and coronal noncontrast T2-weighted images. Postcontrast axial, coronal, and sagittal T1-weighted images are then obtained using fat suppression.
Imaging
Computed tomography (CT) and magnetic resonance (MR) imaging can be considered complimentary diagnostic studies for the evaluation of aggressive inflammatory and neoplastic diseases of the sinuses ( Figs. 1 and 2 ). CT is typically the first diagnostic test used for the evaluation of the paranasal sinuses. With thin slices and multiplanar reformations, CT does a satisfactory job at evaluating most disease processes involving the sinuses. CT is excellent at evaluating the bony anatomy, orbital fat, bony cortex, and for depicting the internal architecture of some fibro-osseous (see Fig. 1 ) and other bony lesions (see Fig. 2 ). MR imaging is a better choice for identifying the full extent of aggressive sinonasal disease that spread into the orbit, skull base, brain, and cavernous sinus. It is more definitive than CT for separating tumor from inflammatory mucosal disease and retained secretions. MR imaging is superior to CT for detecting the full extent of perineural spread of tumors. In many cases, CT and MR imaging play complimentary roles. Important things to evaluate include bone remodeling versus destruction, presence of calcification, inherent T1 and T2 signal intensity, lesion cellularity ( Fig. 3 ), degree of contrast enhancement, vascularity, and the involvement of the orbital, premaxillary, and retromaxillary fat. Detecting spread into the orbit, skull base, cavernous sinus, and brain as well as the presence of perineural tumor spread will usually alter the type of treatment. The accurate assessment of tumor spread will also influence the discussion of viable treatment options and overall disease prognosis with patients. Cerebral angiography or MR angiography may be helpful in the management of vascular sinonasal masses (see Fig. 2 ; Fig. 4 ). The radiologist’s job is to separate nonaggressive inflammatory sinus disease from more aggressive inflammatory or neoplastic processes. Patients can then undergo a biopsy for the final diagnosis and appropriate treatment.
A recommended CT protocol includes the acquisition of thin (0.5–1.5 mm) axial images that are reconstructed in both soft tissue and bone algorithms (see Fig. 2 ). These images are then used to generate thin (1.25–2.5 mm), 2-dimensional reconstructions in the coronal and sagittal planes (see Fig. 1 ). A typical MR imaging protocol includes axial, coronal, and sagittal noncontrast T1-weighed images, axial diffusion-weighted images, and axial and coronal noncontrast T2-weighted images. Postcontrast axial, coronal, and sagittal T1-weighted images are then obtained using fat suppression.
Anatomy
The paranasal sinuses are present to a varying degree in newborns. The maxillary and ethmoid sinuses are usually present at birth, whereas the frontal and sphenoid sinuses are absent. The sinuses undergo varying rates of maturation, with each of the sinuses showing time periods of more rapid development. The paranasal sinuses reach near maturity by 12 years of age, with maximal size attained by early adulthood. Sinus opacification is typically not considered of clinical significance until the age of 2 to 3 years. The paired maxillary sinuses develop within the maxillary bones and contain 4 recesses: alveolar, zygomatic, palatine, and infraorbital. The mucosa is lined by ciliated epithelium that propels secretions to the ostia of each sinus. The drainage pathway of the maxillary sinus is via the ostiomeatal unit, which is centered about the uncinate process and is comprised of the maxillary ostium, infundibulum, hiatus semilunaris, and middle meatus. An accessory ostium may be seen in approximately 20% to 30% of patients. Innervation is via maxillary nerve branches, including the anterior, middle, and posterior superior alveolar nerves. Arterial supply is primarily via the posterior superior alveolar artery and the infraorbital and greater palatine arteries.
The ethmoid sinuses consist of several pneumatized 3-18 air cells within each ethmoid bone. These cells are divided into anterior and posterior cell groups by the basal lamella. The agger nasi cell lies anterior to the frontal sinus drainage pathway and may be a source of frontal sinus obstruction. Additional inconstant air cells include frontal infundibular cells (Bent-Kuhn types 1–4). The lateral border of the ethmoid sinus is formed by the lamina papyracea, which is also the medial wall of the orbits. The roof of the ethmoid sinuses is covered by the fovea ethmoidalis, a projection of the frontal bone. Adjacent to the upper medial wall of the ethmoid sinus is the medial and lateral lamella of the cribriform plate of the ethmoid bone. The appearance of the cribriform plate has been classified into Keros types 1 to 3 according to the depth that it extends into the nasal cavity. Innervation is from the anterior and posterior ethmoid nerves that are branches of the nasociliary nerve, which arises from the V1 portion (ophthalmic division) of the trigeminal nerve. The blood supply is via the anterior and posterior ethmoidal arteries, which are branches of the ophthalmic artery.
The frontal sinus is typically the last sinus to aerate. Up to 4% of the population may not develop a frontal sinus. It is variable in size, ranging from nonpneumatized to hyperpneumatized (involving almost all of the frontal bone). A variable number of bony septations may be present in the frontal sinus. Drainage is via the frontal recesses into the middle meatus. Innervation is from branches of the supraorbital nerves, which are branches of the ophthalmic nerve (V1). The blood supply is from the supraorbital and anterior ethmoidal arteries.
The sphenoid sinus lies within the sphenoid bone just posterior to the nasal cavity. It may contain variable bony septa. The sphenoid sinus can be hypoplastic in size or hyperpneumatized with aeration of the lateral pterygoid recesses and even the greater sphenoid wing. Drainage is via the sphenoid ostia into the sphenoethmoidal recess of the posterosuperior nasal cavity. Several important structures lie in close proximity to the sphenoid sinus. These structures include the internal carotid arteries; cavernous sinuses; and cranial nerves III, IV, V1, V2, and VI. The optic nerves lie superomedial to the sphenoid sinuses. The floor of the anterior cranial fossa, sella turcica, and pituitary gland are located superiorly. The vidian nerve courses along the floor of the sinus. It is occasionally located entirely within the sinus lumen on a thin bony stalk when the pterygoid recess is hyperpneumatized. The foramen rotundum lies along the inferolateral aspect of the sinus. Innervation of the sphenoid sinus is from the posterior ethmoidal nerves and postganglionic parasympathetic fibers of the facial nerve. The blood supply is primarily via the posterior ethmoidal and pterygovaginal arteries.
The pterygopalatine fossa is located posterosuperior to the medial wall of the maxillary sinus and is a central way station for potential perineural spread. It is in communication with the nasal cavity medially via the sphenopalatine foramen, infrazygomatic masticator space laterally via the pterygomaxillary fissure, orbit anteriorly via the superior and inferior orbital fissures, cavernous sinus and middle cranial fossa posteriorly via the foramen rotundum, facial nerve genu posteriorly via the pterygoid canal, and palate inferiorly via the descending pterygopalatine canal.
Lymph node drainage of the sinuses can be through anterior channels, including the facial, parotid, submandibular, and upper internal jugular chain nodes, or posterior drainage to the retropharyngeal nodes. Nodal involvement is most common with maxillary sinus cancers (approximately 15%), uncommon with ethmoid involvement, and almost never seen with sphenoid or frontal sinus tumors.
Tumor staging
Staging of sinus neoplasms is most commonly based on the 2010 American Joint Committee on Cancer (AJCC) staging manual ( Boxes 1–3 ). TNM staging is divided into a maxillary sinus staging and a nasal cavity and ethmoid sinus staging. Frontal and sphenoid sinus cancers are rare enough that no TNM staging exists. Final staging of any malignancy should be a combination of clinical, radiologic, and pathologic staging. Melanoma has a separate staging system, which is discussed later.
T1: Tumor limited to maxillary sinus mucosa with no erosion or destruction of bone
T2: Tumor causing bone erosion or destruction, including extension into the hard palate or middle nasal meatus, except extension to posterior wall of maxillary sinus and pterygoid plates
T3: Tumor invades any of the following: bone of the posterior wall of the maxillary sinus, subcutaneous tissues, floor or medial wall of orbit, pterygoid fossa, ethmoid sinuses
T4a (moderately advanced local disease): Tumor invades anterior orbital contents, skin of cheek, pterygoid plates, infratemporal fossa, cribriform plate, sphenoid or frontal sinuses
T4b (very advanced local disease): Tumor invades any of the following: orbital apex, dura, brain, middle cranial fossa, cranial nerves other than maxillary division of trigeminal nerve (V2), nasopharynx, or clivus
T1: Tumor restricted to any one subsite, with or without bony invasion
T2: Tumor invading 2 subsites in a single region or extending to involve an adjacent region within the naso-ethmoidal complex, with or without bony invasion
T3: Tumor extends to involve the medial wall or floor of the orbit, maxillary sinus, palate, or cribriform plate
T4a (moderately advanced local disease): Tumor invades any of the following: anterior orbital contents, skin of nose or cheek, minimal extension to anterior cranial fossa, pterygoid plates, sphenoid, or frontal sinuses
T4b (very advanced local disease): Tumor invades any of the following: orbital apex, dura, brain, middle cranial fossa, cranial nerves other than maxillary division of trigeminal nerve (V2), nasopharynx, or clivus
Nodal staging a
NX: Regional lymph nodes cannot be assessed
N0: No regional lymph node metastasis
N1: Metastasis in a single ipsilateral lymph node, 3 cm or less in greatest dimension
N2a: Metastasis in a single ipsilateral lymph node, more than 3 cm, but not more than 6 cm in greatest dimension
N2b: Metastasis in multiple ipsilateral lymph nodes, none more than 6 cm in greatest dimension
N2c: Metastasis in bilateral or contralateral lymph nodes, none more than 6 cm in greatest dimension
N3: Metastasis in a lymph node, more than 6 cm in greatest dimension
Distant metastasis
M0: No distant metastasis
M1: Distant metastasis
a A modifier for the presence or absence of extracapsular disease may be used (ECS±). Extracapsular spread does not affect staging.
Maxillary sinus staging historically involved the use of the Ohngren’s line. This line was drawn on a lateral radiograph connecting the medial canthus of the orbit and the angle of the mandible. It divided the maxillary sinus into anteroinferior and posterosuperior segments. Posterosuperior disease had a poorer prognosis because of its closer proximity to the skull base, orbit, and other critical structures. Current TNM staging is depicted in Boxes 1–3 .
The assessment for lymph node involvement includes evaluating size, shape, central or peripheral hypodensity, and enhancement, and the evaluation for the presence or absence of extracapsular extension. The AJCC nodal staging system for sinus neoplasm is depicted in Box 3 . Note that N3 nodal disease requires the enlargement of a single node and not a conglomerate of multiple nodes. Imaging assessment for tumor spread to nodes is difficult in daily practice with sensitivity and specificity in the moderate range. Sensitivity and specificity measurements based on a nodal size of 10 mm, in one study, were 88% and 39% for CT and 81% and 48% for MR imaging. It is, therefore, important to use other imaging signs of nodal metastasis (enhancement, central hypointensity, T1- or T2-weighted signal inhomogeneity) in addition to nodal size when calling a node positive. In practice, the decision as to whether or not to treat the neck for nodal spread will not be completely based on imaging findings or clinical examination alone. If the probability for nodal disease involvement for a given T-stage of a tumor is high (>20%–30%), the neck will often be treated electively regardless of whether there is clinical or radiographic evidence of nodal spread. Positron emission tomography (PET) imaging can be helpful in the characterization of the primary tumor and any metastatic nodes. The accuracy of nodal assessment by PET compared with CT and MR imaging is approximately 67% to 90% sensitive and 81% to 94% specific for PET and CT and MR imaging are approximately 64% to 82% sensitive, 75% to 85% specific and 67% to 80% sensitive, 77% to 79% specific, respectively. Similarly, PET is useful for the pretreatment detection of distant metastasis in high-risk patients, evaluation of posttreatment response of the primary tumor, and for subsequent posttreatment surveillance for recurrence. Posttreatment PET is preferably performed at least 12 weeks after therapy to help minimize the postsurgical and postradiation inflammatory changes that can lead to false-positive results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree