Chapter Outline
Magnetic Resonance Imaging Technique
Magnetic resonance imaging (MRI) is frequently used as a problem-solving tool for the evaluation of disease processes throughout the body and is a powerful imaging modality to characterize disease in the solid abdominal organs. With widespread availability, technologic advances, and increasing familiarity of ordering physicians and radiologists alike, MRI is becoming the modality of choice for parenchymal lesion detection, anatomic localization, and lesion characterization. MRI has high spatial and temporal resolution without exposure to ionizing radiation, allowing accurate imaging on a wide spectrum of patients, including pregnant women and children.
Various technologic advances in MRI have been made in recent years, including increased magnetic strength, improved coil technology, and advanced imaging sequences. The purpose of this chapter is to discuss commonly used imaging sequences and protocols as well as recent developments in advanced sequences in MRI of the abdomen. An all-encompassing summary of each pathologic diagnosis in the abdomen is beyond the scope of this chapter; the chapter reviews solid abdominal organ lesions and pathologic processes in which MRI is crucial for accurate diagnosis and characterization.
Magnetic Resonance Imaging Technique
Field Strength
Of the recent advances in MRI, the most significant is the increasing magnetic field strength. The most common field strengths in clinical use in MRI are 1.5T and 3.0T. Higher magnetic field strengths allow increased longitudinal magnetization and signal intensity, resulting in increased signal-to-noise ratio (SNR). The resulting increased SNR can be used to increase spatial resolution or to decrease imaging acquisition time, allowing improved imaging.
There are disadvantages to higher magnetic field strengths. Higher purchasing prices in addition to higher maintenance prices are among the disadvantages. Reduced T1 contrast secondary to lengthening of T1 is a limitation of higher magnetic field strengths. Increased susceptibility from magnetic field inhomogeneity, patient motion, and chemical shift are also a disadvantage of higher magnetic field strengths.
Surface Coils
For imaging of the abdomen, the goal is to maximize SNR for a large area of interest, and a multichannel phased-array body coil is typically used. Flexible coils are used to maintain the contour of the torso, which allows high-resolution imaging of the abdomen and pelvis. Phased-array coils allow parallel imaging, in which each channel has its own receiver, and the multichannel system increases the number of receiver coils. Advances in phased-array coil technology have resulted in increased SNR and improved image quality, faster scan time, and larger scan area.
Other body parts, such as the rectum or the female pelvis, can be imaged with a phased-array body coil that covers a smaller field of view with high-resolution images. Intraluminal coils are also used to image pelvic organs such as the prostate gland, yielding images with high SNR and high resolution. Anatomy, extracapsular extension, perivascular invasion, and lymphadenopathy can be readily assessed by MRI of the prostate.
Magnetic Resonance Sequences
The basic MR sequences employed in imaging of solid abdominal organs are fairly standard. These include axial and coronal T2-weighted images with and without fat suppression, diffusion-weighted images with apparent diffusion coefficient (ADC) maps, in- and out-of-phase gradient-echo images, and dynamic contrast-enhanced three-dimensional T1 gradient-echo images with fat saturation. Two- and three-dimensional free-breathing navigator-triggered magnetic resonance cholangiopancreatography (MRCP) sequences may also be obtained if clinically indicated. Additional sequences, such as balanced fast field echo or fast imaging employing steady-state acquisition sequences, can be obtained for evaluation of vascular anatomy, delineation of bowel and mesentery, and quantification of fat by T2*.
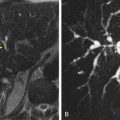
Single-shot T2-weighted images are rapidly acquired, heavily T2-weighted images that are useful for evaluating fluid and structures with short T2 values. The benefit of this sequence is the speed with which images are acquired; however, the contrast-to-noise ratio is not as high as in other T2-weighted fast spin-echo sequences. Axial T2 fast spin-echo sequences with fat suppression have the benefit of increased liver-to-spleen contrast and are useful for detection of lesions. Lymph nodes are also easily detected on this sequence. PROPELLER (periodically rotated overlapping parallel lines with enhanced reconstruction) is a fast imaging technique whereby a fraction of k space is acquired in each frame. Each imaging acquisition in a PROPELLER sequence acquires a rectangular portion of k space, with each acquisition acquiring images through the k space center, and can reduce artifact from respiratory motion ( Fig. 67-1 ). Other T2-weighted sequences, such as the three-dimensional turbo spin-echo sequence known as SPACE (sampling perfection with application-optimized contrast with different flip-angle evolutions), have been developed to decrease image acquisition times. With this sequence, the acquired images serve as a data set that can be used to create postprocessed reformatted images in axial, coronal, and sagittal planes. The SPACE sequence decreases the number of T2-weighted image acquisitions and can potentially replace two-dimensional T2-weighted images.

Diffusion-weighted imaging with varying diffusion gradient strengths has become important for evaluating malignant neoplasms and inflammatory conditions. The principle of diffusion-weighted imaging is based on random motion of microscopic water molecules and diffusion of water in body tissues. Diffusion of water molecules is based on cell membrane integrity and tissue cellularity. Diffusion-weighted imaging provides functional information about tissue cellularity along with the ADC values, which are calculated from diffusion-weighted imaging data. An increase in the number of cells in conditions such as primary malignant neoplasms, metastases, and infection or abscesses leads to crowding of extracellular spaces and further restriction of water molecules.
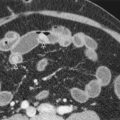
T1-weighted in-phase and opposed-phase gradient-echo images are useful for detection of focal and diffuse hepatic steatosis, but the T2* effects and susceptibility artifact are also useful components of this sequence. There is signal loss on the opposed-phase images at water-fat interfaces, resulting in the India ink artifact or fat-water cancellation artifact, whereby a sharply defined black rim is seen at water-fat interfaces. Water and fat protons precess at different frequencies, and in spin-echo sequences, a 180-degree refocusing pulse causes the protons to be in phase when the echo is acquired; however, gradient-echo images lack the 180-degree refocusing pulse. Focal and diffuse hepatic steatosis is manifested as loss of signal on the opposed-phase images ( Fig. 67-2 ). The dual gradient-echo images also provide information by T2* effects, whereby hepatic parenchymal storage diseases, such as hemochromatosis and hemosiderosis, decrease in signal intensity with the longer time to echo secondary to continued transverse magnetization decay ( Fig. 67-2 ). Susceptibility artifacts result from field inhomogeneities occurring at interfaces of materials with varying magnetic susceptibility, such as metallic objects, gas-filled bowel, and pneumobilia ( Fig. 67-3 ). The difference in precession rates of fat and water protons is proportional to the magnetic field strength and is 3.5 ppm or 225 Hz at 1.5T. At 3T, the difference in precession rates is 450 Hz, and the protons are out of phase at 1.1 ms, 3.4 ms, 5.6 ms, and so forth, whereas the protons are in phase at 2.2 ms, 4.5 ms, 6.7 ms, and so forth. Acquiring the out-of-phase image at a short time interval of 1.1 ms followed by the in-phase image at 2.2 ms can be challenging, and the second- or third-order echo may need to be obtained. The DIXON method uses the T1 in-phase and out-of-phase images to mathematically calculate “fat-only” and “water-only” images, which is particularly useful in areas with high magnetic susceptibility ( Fig. 67-4 ).



Dynamic contrast-enhanced three-dimensional T1-weighted gradient-echo images with fat saturation sequences are helpful in assessing for the presence of enhancement as well as characteristic enhancement patterns of certain lesions. Dynamic imaging with arterial, portal venous, equilibrium, and delayed phases is useful for evaluation of all upper abdominal organs, including the liver, spleen, pancreas, adrenal glands, and kidneys. Images are performed with a breath hold of approximately 15 to 20 seconds. Subtraction images are obtained by subtracting the images made before the administration of gadolinium from the dynamic contrast-enhanced images and are helpful in assessing for the presence and degree of enhancement.
Two- and three-dimensional free-breathing navigator-triggered MRCP sequences have excellent spatial and temporal resolution. Accurate assessment of the hepatobiliary and pancreatic ducts with MRCP has replaced endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography as the initial study of choice in the evaluation of hepatobiliary and pancreatic ducts.
Magnetic Resonance Contrast Agents
Multiple MR contrast agents are currently in use. They can be categorized into five classes: extracellular, hepatobiliary, reticuloendothelial, blood pool, and combined agents. A complete list and thorough review of the mechanism of action and side effects are beyond the scope of this chapter; however, the indications for and MR techniques used to enhance these agents are briefly reviewed.
Extracellular agents such as gadopentetate dimeglumine (Magnevist) have been in use for the longest time clinically. These agents are distributed in the extracellular space; 20 mL is administered and nearly entirely excreted by the kidneys.
Hepatobiliary agents such as gadoxetic acid (Eovist) are taken up by hepatocytes and excreted through the biliary system, which causes the liver, biliary ducts, and hepatocyte-containing lesions to be T1 hyperintense. The average dose is 0.025 mmol/kg for approximately 10 mL, and there is around 50% hepatic and 50% renal excretion. The delayed hepatobiliary phase ranges from 10 minutes to hours, which is significantly shorter compared with the combined agent gadobenate dimeglumine (MultiHance).
Blood pool agents stay intravascular for a longer time compared with the extravascular agents and are used for angiography. Although use of blood pool agents is limited in imaging of solid abdominal organs, it is listed here for completeness.
Combined agents have extracellular, blood pool, and hepatobiliary characteristics. Gadobenate dimeglumine (MultiHance) is taken up by hepatocytes, and 5% of the administered dose is excreted in bile; the remaining 95% is excreted renally. A limitation of this agent is that the hepatobiliary phase occurs approximately 1 to 2 hours after ingestion of the contrast agent, which is significantly longer compared with hepatobiliary agents.
Imaging of Abdominal Organs
A complete summary of each pathologic diagnosis in the abdomen is beyond the scope of this chapter, and as such, this chapter reviews solid abdominal organ lesions for which classic MRI characteristics are diagnostic. MRI features are crucial for narrowing the differential diagnosis and making accurate diagnoses.
Liver
The three most common benign hepatic lesions encountered are cavernous hemangiomas, focal nodular hyperplasia (FNH), and hepatic adenomas. FNH and adenomas share imaging characteristics but can be accurately differentiated by various MR sequences and contrast agents. FNH can also be differentiated from fibrolamellar hepatocellular carcinoma (HCC) on the basis of MR characteristics.
Cavernous Hemangiomas
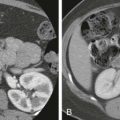
Cavernous hemangiomas, the most common primary liver tumor, occur in 20% of the general population. On histologic examination, hemangiomas are composed of large blood-filled vascular channels of varying sizes and shapes. Typically, they are solitary and small; however, multiplicity can occur, and giant cavernous hemangiomas can be larger than 20 cm. On imaging, hemangiomas have characteristic appearances. On ultrasound (US), hemangiomas are focal, homogeneous, and echogenic and can have varying appearances, depending on the degree of background hepatic steatosis, which can be easily assessed on MRI. MRI shows typical features of hemangiomas, which include T1 hypointensity and marked T2 hyperintensity; on the dynamic contrast-enhanced images, hemangiomas have peripheral nodular enhancement with progression in a centripetal distribution ( Fig. 67-5 ). Flash-filling hemangiomas can be seen, in which the entire lesion homogeneously enhances in the arterial phase. In addition, the very bright T2 hyperintense signal has a high sensitivity (100%) and specificity (92%) for differentiation of hemangiomas from hepatic metastases.

Focal Nodular Hyperplasia
FNH is the second most common benign hepatic tumor after hemangiomas. It is an aggregate of hepatocytes that form in response to a “central scar,” which represents an underlying vascular malformation or clusters of blood vessels and bile ducts. FNH is hypointense to isointense on T1-weighted images and isointense to mildly hyperintense on T2-weighted images with a T2 hyperintense scar. After the administration of gadolinium, there is brisk, homogeneous, and intense arterial enhancement of the lesion with the exception of the central scar, which has gradual delayed enhancement ( Fig. 67-6 ).

Approximately 20% of FNH cases are atypical and do not have characteristic imaging features. The three subtypes include telangiectatic FNH, FNH with cytologic atypia, and mixed hyperplastic and adenomatous FNH, all of which do not possess the central vascular malformation and are often challenging to diagnose on imaging. These three nonclassic FNH types have bile ducts and some degree of normal hepatocytes, allowing uptake and excretion of hepatobiliary contrast agents.
The delayed enhancement of the central scar helps differentiate FNH from fibrolamellar HCC, which is an uncommon type of HCC. Fibrolamellar HCC usually is manifested as a large heterogeneous T1 hypointense and T2 hyperintense mass, and a majority (80%) of fibrolamellar HCCs contain a true central scar ( Fig. 67-7 ). In contradistinction to FNH, the central scar is a true scar that is T2 hypointense and demonstrates minimal or no enhancement after gadolinium administration.

Hepatic Adenoma
Hepatic adenomas are rare benign tumors containing well-differentiated hepatocytes with few or no biliary ducts. Most adenomas are single, and 90% of adenomas occur in women. On the basis of recent pathologic and genetic evaluation of hepatic adenomas, four subtypes of adenomas have been developed: inflammatory hepatocellular adenoma, hepatocyte nuclear factor 1α (HNF-1α)–mutated hepatocellular adenoma, β-catenin–mutated hepatocellular adenoma, and unclassified. Each type should be considered a different entity because imaging appearances as well as management options vary according to the subtype.
Inflammatory adenomas were previously known as telangiectatic adenomas or telangiectatic FNH. This subtype commonly occurs in young women taking oral contraceptive medications and obese patients. On MRI, inflammatory adenomas are T1 isointense to mildly hyperintense and T2 diffusely intense to hyperintense. T1-weighted in-phase and opposed-phase gradient-echo images show little or no loss of signal. After the administration of gadolinium, inflammatory adenomas enhance on the arterial phase and persist on the portal venous and delayed phases ( Fig. 67-8 ). Two complications of hepatic adenomas are intratumoral bleeding and development of HCC. Bleeding occurs in 25% to 50% of adenomas, and tumors larger than 5 cm are at increased risk for bleeding and rupture. Inflammatory adenomas have the highest risk of bleeding of all subtypes.

HNF-1α–mutated hepatocellular adenoma accounts for 30% to 35% of all adenomas and is the second most common subtype. This subtype occurs almost exclusively in women, with the majority of patients taking oral contraceptive medications. Estrogens in these medications have been postulated to cause somatic mutations in HNF-1α adenomas, and inactivation of HNF1A gene results in nonfunctioning HNF-1α protein, which subsequently promotes proliferation of hepatocytes and lipogenesis with impaired fatty acid regulation in hepatocytes, resulting in fat deposition within the hepatocytes. On MRI, HNF-1α–mutated hepatocellular adenomas are T1 hyperintense to isointense and T2 isointense to slightly hyperintense; after the administration of contrast material, there is moderate arterial enhancement with no persistent portal venous or delayed phase enhancement. Diffuse signal loss on out-of-phase gradient recalled echo images is seen because of intracellular steatosis, with sensitivity and specificity as high as 85% and 100%, respectively. HNF-1α–mutated hepatocellular adenomas, even lesions larger than 5 cm, have minimal risk of bleeding, and there is minimal or no risk for development of HCC.
β-Catenin–mutated hepatocellular adenoma accounts for 10% to 15% of all adenomas. This subtype occurs more commonly in men and is associated with exogenous testosterone use, familial adenomatosis polyposis, and glycogen storage disease. There is overexpression of the glutamate-ammonia lipase gene, resulting in elevated levels of glutamine synthetase, which has been used recently in immunohistochemical analysis for definitive diagnosis. This subtype has homogeneous or heterogeneous T1 and T2 hyperintense signal, depending on the presence of blood and necrosis. These lesions enhance intensely on the arterial phase, with variable persistence on the portal venous and delayed phases. The imaging appearance can be confused with that of HCC. This subtype has the highest risk of malignant transformation, with an overall risk of 5% to 10% as the β-catenin pathway is involved in the pathogenesis of HCC. Other risk factors for malignant transformation are male sex, glycogen storage disease, tumors larger than 5 cm, and exogenous anabolic steroid use.
Focal and Diffuse Steatosis
Various manifestations of fatty infiltration include focal, patchy, and diffuse. Focal fat deposition and focal fat sparing can be mistaken for a focal hepatic lesion. MRI is helpful in differentiating a true hepatic lesion from focal fat deposition and focal fat sparing, particularly the T1-weighted in-phase and opposed-phase gradient-echo images. Focal fat deposition is lower in intensity on the opposed-phase images compared with the in-phase images and has high signal on the DIXON fat-only images (see Fig. 67-4 ). In contradistinction, focal fat sparing does not decrease in intensity on the opposed-phase images but instead appears hyperintense relative to the surrounding hepatic steatosis.
Another helpful differentiating imaging feature is the location, which typically is in the gallbladder fossa, in the medial aspect of the left hepatic lobe, along the falciform ligament, and in the central aspect of segment IV. Also, no mass effect is seen on adjacent structures or hepatic parenchyma, and blood vessels course through areas of focal hepatic steatosis and focal fat sparing. The presence of normal enhancing liver is helpful in accurate diagnosis of focal fat and focal fat sparing.
Diffuse hepatic steatosis involves a majority of the hepatic parenchyma, and on MRI, the liver intensity decreases on the opposed-phase images relative to the in-phase images (see Fig. 67-2A, B ). Rather than appearing as focal hepatic signal abnormality, the entire hepatic parenchyma is diffusely involved.
Cholangiocellular Origin Lesions
MRI is extremely helpful in diagnosing and characterizing lesions of cholangiocellular origin, including simple hepatic cysts, biliary hamartomas, peribiliary cysts, biliary cystadenoma, and biliary cystadenocarcinoma. MRCP sequences are useful to assess the relationship of these lesions to the biliary tree.
Simple hepatic cysts are lined by biliary epithelium and originate from precursor microhamartomas. Cysts are usually round, well defined, and larger than 1 cm, and they can be single or multiple. Multiple simple hepatic cysts may be seen in adult polycystic kidney disease. On MRI, simple hepatic cysts have classic imaging characteristics with T1 hypointensity, T2 hyperintensity, and no associated enhancement.
Biliary hamartomas are also known as von Meyenburg complexes. They represent rare benign malformations of the biliary system secondary to failed involution of embryonic biliary ducts. Hamartomas are usually numerous, subcentimeter, well-defined T1 hypointense, T2 hyperintense, nonenhancing lesions with no connection to the biliary tree ( Fig. 67-9A ).

Peribiliary cysts are benign cystic dilations of obstructed glands around the biliary duct walls. Inflammation and disturbed periportal circulation lead to gland obstruction and result in peribiliary cyst formation. These lesions are typically associated with cirrhosis with portal hypertension and portal vein thrombosis. Peribiliary cysts are usually multiple T1 hypointense, T2 hyperintense tubular structures coursing along the bile ducts ( Fig. 67-9B ). They typically have smooth walls with no internal components, and they do not communicate with the biliary system.
Biliary cystadenoma, a rare benign cystic neoplasm, is a precursor to the malignant form of biliary cystadenocarcinoma. A majority of these lesions originate from the intrahepatic bile ducts and are complex, multiloculated, cystic masses with enhancing internal septations ( Fig. 67-9C ). The MRI findings are variable, depending on the internal composition of the lesion, specifically if the lesion is composed of mucinous or serous fluid. The presence of enhancing nodular septations in the lesion is suggestive of the malignant biliary cystadenocarcinoma. These tumors tend to recur after complete surgical excision, and biliary cystadenomas can undergo malignant degeneration even after years of stability.
Cirrhosis and Hepatocellular Carcinoma
Cirrhosis is associated in up to 90% of patients with HCC and is characterized by irreversible disruption of the normal hepatic architecture with fibrosis and hepatic nodules. The most common causes of cirrhosis worldwide, in order of decreasing frequency, are hepatitis C virus, hepatitis B virus, alcohol, and nonalcoholic steatohepatitis; in the United States and Europe, the most common cause of cirrhosis is alcohol. Hepatitis B and C increase the risk of HCC 20 times, and the rate of HCC development once a diagnosis of cirrhosis is made is 1% to 4% per year.
The development of HCC is a multistep continuous carcinogenic process whereby multiple alterations lead to malignant transformation of the hepatocyte. The hepatocarcinogenesis has been arbitrarily divided for diagnostic and practical purposes. There are two types of hepatocellular nodules, regenerative and dysplastic nodules. Regenerative nodules result from proliferation of hepatocytes and supporting stroma, leading to nodular transformation of hepatic parenchyma with no fibrous septations between the nodules. These nodules can be monoacinar or multiacinar and can also be micronodular (<3 mm) or macronodular (>3 mm). Dysplastic nodules result from hepatocyte proliferation with abnormal growth or dysplasia, with derangement of normal architecture and cellular atypia. HCC is a malignant neoplasm with hepatocellular differentiation and development of neoangiogenesis with progressive loss of portal venous blood flow and increased arterial vascularity.
Cirrhosis on imaging is characterized by atrophy and hypertrophy of certain segments, liver contours, presence of hepatic nodules, fibrotic and fatty changes, and signs of portal hypertension. The right lobe of the liver and the medial segment of the left hepatic lobe (segment IV) atrophy, whereas the caudate and lateral segments of the left hepatic lobe (segments II and III) hypertrophy. The liver contour tends to be nodular, although this may not be apparent in all cirrhotics, and there is widening of the fissures between lobes and segments. Regenerative nodules are seen, and diffuse lacelike bands of fibrosis may be present; diffuse or geographic areas of fatty change have also been seen in cirrhosis. Signs of portal hypertension, including splenomegaly, enlargement of the portal vein, presence of varices (gastric, splenic, esophageal, caput medusae), and peribiliary cysts, are present in varying degrees in cirrhotic patients.
MRI has revolutionized the diagnostic and treatment implications involved in the care of cirrhotic patients. MRI can accurately characterize cirrhosis-associated liver nodules, with the critical role being early detection of HCC. The MRI examination is easily and accurately reproducible, allowing staging of HCC, planning of treatment options, and assessment of response to treatment. HCC is T2 hyperintense with avid arterial enhancement that shows washout or becomes hypointense relative to the surrounding liver on the delayed phase ( Fig. 67-10 ).