The management of Crohn disease (CD) has entered a new era in which it is imperative to incorporate objective data and symptoms to plan the best therapeutic approach for each patient. Magnetic resonance (MR) imaging provides powerful objective insights into the disease process, and its high sensitivity and specificity for detecting inflammation make it essential for diagnosis and management. Growing evidence indicates that MR provides reliable and accurate information that enables detection of changes after treatment with biological drugs. This article provides an overview of currently established and emerging MR biomarkers for assessing response to treatment in patients with CD.
Key points
- •
Magnetic resonance (MR) enterography has a key role in both clinical research and practice.
- •
Using cross-sectional imaging to predict the response to treatment is essential to tailoring treatment.
- •
Objective and reproducible indexes based on MR findings enable disease severity to be graded, making it possible to assess therapeutic efficacy.
- •
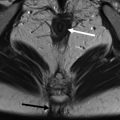
Pelvic MR imaging is the gold standard for monitoring patients with perianal Crohn disease and helps optimize treatment.
Introduction
In Crohn disease (CD), the clinical decision making and new drug evaluation processes are based mainly on symptom control. Clinicians order changes to treatment to better manage symptoms associated with inflammatory activity (eg, diarrhea and abdominal pain), impairment of general well-being, or extraintestinal manifestations. The measurements of clinical symptoms alone, for example, by using the Crohn’s Disease Activity Index (CDAI), are not a reliable tool, however, to monitor the course if the disease. An analysis of data from the Study of Biologic and Immunomodulator Naive Patients in Crohn Disease (SONIC) trial showed that 18% of patients classified by the CDAI as having moderate to severe disease (CDAI >220) had no ulcers at endoscopy. Moreover, after therapeutic intervention, 47% of patients who were classified as in remission by the CDAI (CDAI <150) still had ulcers, and 35% of those whose mucosa had healed were classified as still having active disease.
Imaging, either cross-sectional imaging or endoscopic, has become the standard of care for initial diagnosis of CD and for further follow-up by assessing severity and detecting complications. Clinical symptoms, although relevant for detecting flare-ups of the disease, are subjective, and more objective tools, such as imaging, for detecting active disease are needed.
The therapeutic armamentarium in CD has greatly expanded in the past 2 decades and up to a dozen biologics drugs and immunomodulators are available for modern inflammatory bowel disease (IBD) care. The approach to therapeutics has also changed with a more aggressive approach using a treat-to-target philosophy. The most relevant target used in practice and in research is mucosal healing. The healing of ulcerations has been associated with better outcomes in terms of reduction of patient hospitalization and need of surgical resections. Therefore, for guiding therapeutic decisions in patients with CD. it is essential to have accurate and reliable tools to demonstrate healing of inflammatory bowel lesions.
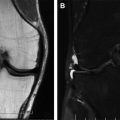
Magnetic resonance enterography (MRE) offers several advantages over other imaging modalities for use in assessing the status of the disease and for the monitorization of CD. MRE enables the assessment of disease activity, which is out of reach of the endoscope (transmural or isolated small bowel disease), which may facilitate the evaluation of the whole small bowel. MRE-based assessment might be more responsive to endoscopic activity indices, such as the Simplified Endoscopic Score for Crohn’s Disease (SES-CD), which is subject to colonic preparation and technical difficulties. Therefore, in clinical research, for assessment of efficacy outcomes, MRE again ensures assessment of all intestinal segments and avoids operator-dependent variability between baseline and post-treatment assessments. In clinical practice, MRE provides information that changes clinician perspectives and also influences planned therapeutic strategy in a significant proportion of patients. Finally, unlike computed tomography enterography, MRE does not require exposure to ionizing radiation, which is a key aspect considering the general young age at the time of diagnosis and the fact that patients require multiple reassessments of the disease during their life.
In the clinical scenario of perianal fistulizing CD, pelvic magnetic resonance imaging (MRI) has been shown to be a highly accurate noninvasive modality for the diagnosis and classification of perianal fistulas. In addition, pelvic MRI provides detailed information on fistula anatomy, extensions, severity, and presence of perianal collections that is key to planning the best therapeutic approach, usually requiring surgery followed by medical treatment. Currently, pelvic MRI it is considered the gold standard imaging technique for perianal CD.
For the short-term and long-term monitorization of the severity of the disease, in particular in clinical research, the use of quantitative biomarkers allows the conversion of MRE-based observation into a statistically tractable framework. In that regard, the multiparametric nature of MRI has allowed the development of different magnetic resonance (MR)-based indexes using regression models and a valid reference standard. This article reviews the current available biomarkers for measuring the severity of luminal inflammation in CD and discusses the strengths and limitations of each of them. Also, an overview is provided on the scores available for grading the severity of perianal CD based on MRI observations.
Indices for small and colon activity assessment
The derivation of MR-based scores derived from regression models that predict activity assessed by a valid external gold standard (histology or endoscopy) is more stringent than those derived based on expert opinion because only the variables with independent predictor value for severity and activity are selected, whereas those without a proved independent predictor value are excluded from the scoring system. The downside of using tools derived using regression models is that the derived indexes may not be easily applicable in the daily clinical practice if they entail the quantitative measurement of too many or too difficult descriptors.
Magnetic Resonance Index of Activity Score
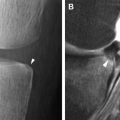
The MR index of activity (MaRIA) is a composite score that takes into account the bowel wall thickness, relative contrast enhancement on the bowel wall before and after gadolinium injection, presence of ulcers, and presence of mural edema (defined as hyperintensity on T2-weighted sequences). The MaRIA was derived as a simplified score to quantify disease activity from MRE findings in each segment of the terminal ileum or the colon:
The global MaRIA score provides information at patient level and is computed as the sum total of the subscores of each intestinal segment.
The MaRIA score provides a continuous and quantitative index of inflammation severity that has been applied in different studies and currently is the best characterized score to grade disease activity using MRE. Two cutoffs were selected in a cross-validation study using ileo-colonoscopy as gold standard, a MaRIA subscore of greater than or equal to 7 for depicting segments with active disease (received operating characteristic [ROC] area under the curve [AUC] = 0.933; sensitivity 0.87 and specificity 0.87) and a subscore greater than or equal to 11 units for identifying segments with severe disease activity (ROC AUC = 0.965; sensitivity 0.92 and specificity 0.92). Moreover, the MaRIA was devised to be highly concordant with the objective endoscopic score, the SES-CD, in the deep small bowel intestine. For these reasons, including through the contribution of the MRE in detecting CD complications, such as strictures, fistula, and abscess, this index can be used as prognostic biomarker to select and assess patient for inclusion in clinical trials.
Besides diagnostic accuracy and responsiveness, reproducibility is a critical property of an evaluative instrument, especially when serial measurements are required. In this sense, the MaRIA has shown almost perfect intrarater reproducibility when was read by central readers, supporting its use as a reliable tool in drug development trials.
The MaRIA score was used to determine the responsiveness of MRE after induction treatment in CD patients after medical therapeutic intervention (after either corticoids or tumor necrosis factor [TNF] inhibitors), showing that the MaRIA score at segment level is sensitive for detecting healing after medical intervention and reliable in detecting the persistence of ulcerations (overall accuracy of 90% for detecting endoscopic mucosal healing) ( Table 1 ).
| Context of Use/Characterization | Publication | Comments/Evidence |
|---|---|---|
| MRE and definition of score and patient selection | Rimola et al, 2009 | Derivation of the MaRIA score highly correlated to CDEIS/endoscopy |
| Rimola et al, 2011 | Validation of the MaRIA correlation with CDEIS/endoscopy | |
| Takenaka et al, 2015 | High concordance between MaRIA and SES-CD; MaRIA accurate at detecting inflammatory activity in small bowel proximal to terminal ileum | |
| Therapeutic response assessment | Ordás et al, 2014 | Concordance between MRE and endoscopy assessment of response to TNF inhibitors and corticosteroids in the terminal ileum and colon |
| Stoppino et al, 2016 | Concordance between MRE and endoscopy assessment of response to TNF inhibitors in the terminal ileum and colon | |
| Takenaka et al, 2018 | Concordance between MRE and enteroscopy assessment of response to TNF inhibitors in the small bowel | |
| Prognosis | Buisson et al, 2017 | Achieving transmural healing according to MaRIA score was associated with longer period in corticosteroid-free remission ( P = .0001) and no needed surgery within 2 y ( P = .0332) |
| Reproducibility | Tielbeek et al, 2013 | Inter-reader reliability |
| Coimbra et al, 2016 | Test-retest, intrareader, and inter-reader reliability | |
| Jairath et al, 2018 | Test-retest, intrareader, and inter-reader reliability |
These observations were externally validated by other groups. In a similar study, Stoppino and colleagues reported on observations of global MaRIA change in response to anti-TNF at week 26 in a cohort of 27 patients with CD and suggest that the optimal cutoff point for identification of patients who had achieved mucosal healing at week 26 was 30.8. In contrast to the original global MaRIA, including 6 segments, in the work of Stoppino and colleagues, global MaRIA was scored as the summation of only 5 segments. This disagreement, however, may be a point of attention suggesting that more stringent criteria for detecting remission at patient level should be used. In that regard, for patients to be in remission, all segmental MaRIA scores must be less than 7. Overall, these results indicate that the MaRIA index can be used for the monitorization of changes over time.
More recently, a Japanese group explored the diagnostic yield of the MaRIA score for detecting healing of lesions in the small bowel using enteroscopy as a reference standard, reporting a similar and high accuracy in both the terminal ileum and in the proximal segments of the small bowel (see Table 1 ).
Despite the wide use of the MaRIA score, the authors acknowledge this index has some limitations. The main drawback is that normal bowel segments contribute to the global MaRIA score whereas ideally normal segments should be scored as zero. As a consequence, in patients with resected segments, an underestimation of the global score occurs. Regarding the acceptance for its calculation, although it takes into account only 4 items, its computation requires measurement of different quantitative parameters (wall thickness and relative contrast enhancement), which is, as a result, time consuming because it must be calculated segment by segment. Hence, a simpler MR tool with faster assessment for measuring disease activity in CD, called simplified MaRIA, has been recently released and further validation in independent cohorts is still required ( Fig. 1 ).

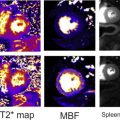
Diffusion-weighted Imaging, Apparent Diffusion Coefficient, and the Clermont Score
The Clermont score (or diffusion-weighted imaging [DWI]-MaRIA) is similar to the MaRIA index, but it uses the apparent diffusion coefficient (ADC) value, calculated by means of the DWI sequence, instead of relative contrast enhancement. To derive and validate the DWI-MaRIA score, the same MRE (by means of the MaRIA score) was considered the reference standard, resulting a new index where wall thickness, mural edema, and ulcerations are included as independent predictors for activity, together with the ADC. The formula to calculate the segmental activity according to Clermont score is as follows:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree