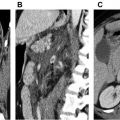
Dynamic contrast-enhanced magnetic resonance lymphangiography is a novel technique to image central conducting lymphatics. It is performed by injecting contrast into groin lymph nodes and following passage of contrast through lymphatic system using T1-weighted MR images. Currently, it has been successfully applied to image and plan treatment of thoracic duct pathologies, lymphatic leaks, and other lymphatic abnormalities such as plastic bronchitis. It is useful in the assessment of chylothorax and chyloperitoneum. Its role in other areas such as intestinal lymphangiectasia and a variety of lymphatic anomalies is likely to increase.
Key points
- •
Dynamic contrast-enhanced magnetic resonance lymphangiography is a novel technique to image central conducting lymphatics performed by injecting gadolinium-based contrast agent into groin lymph nodes.
- •
It includes both T2-weighted imaging and postintranodal injection T1-weighted imaging.
- •
Current applications include assessment of plastic bronchitis in patients with Fontan surgery, chylothorax, chyloperitoneum, and intestinal lymphangiectasia.
Introduction
The lymphatic system is an important component of the circulatory system with essential physiologic functions including nutrition, fluid balance, immunity, and clearing of waste products. It returns excess interstitial fluid and protein to the systemic circulation. Along with lymphoid tissues such as lymph nodes and spleen, it plays an important role in the immunity and clearance of cellular debris including bacteria and proteins. The lymphatic system is also a transporter of long-chain lipids absorbed from the intestine and proteins synthesized in the liver.
Lymph constitutes the excess of tissue fluid, which is derived from blood plasma and removed from the interstitial tissue via the lymphatic system. Lymph contains nutrients, hormones, fatty acids, toxins, and cellular waste products. The most commonly manifested clinical features of lymphatic system disorders are lymphedema, ascites, and pleural effusion, which result from accumulation of tissue fluids due to impaired lymphatic drainage. However, in recent years, the lymphatic system has been implicated in various diseases including inflammatory bowel disease, cancer, and cardiovascular disorders.
Most of the lymphatic system consists of a network of small vessels; hence it is difficult to image it and especially to introduce contrast media into these small lymphatic ducts. However, in the last few years, the lymphatic imaging has been advanced by combining soft tissue contrast and resolution offered by MR imaging, supplemented by the injection of contrast media via a lymph node. These recent advances in imaging methods, along with the development of newer lymphatic interventional techniques have prompted progress in the imaging and treatment of lymphatic pathologies. These advances have focused on the visualization of the central conducting lymphatics (CCLs) such as the cisterna chyli and thoracic duct by dynamic contrast-enhanced magnetic resonance lymphangiography (DCMRL) as well as in the imaging of the extremity’s lymphatic system.
In this review, the authors discuss the anatomy of the lymphatic system and various methods of imaging of the lymphatic system and focus on the DCMRL technique along with its current and potential clinical applications.
Anatomy of the lymphatic system
The lymphatic system consists of a network of small terminal lymphatic ducts and larger lymphatic ducts, with lymph nodes interspersed throughout the lymphatic pathways. Terminal lymphatics are small lymphatic vessels originating in tissues of the body. The lymph collected by terminal ducts from tissues is drained into larger lymphatic ducts that in turn drain into the veins. The lymphatic ducts contain valves, similar to veins, for unidirectional lymph flow from tissues and organs into the systemic venous system. Lymph enters lymph nodes via afferent lymphatic vessels and leaves via efferent lymphatic ducts. Lymph nodes regulate the composition of lymph and mount an immune response if pathogens are detected.
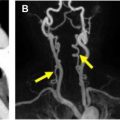
The lymphatic vessels from the right side of the head and neck; right upper extremity; and right side of the chest, lung, and heart drain into the right lymphatic duct. The right lymphatic duct is short (approximately 1.25 cm) and opens directly into the venous system at the junction of the right subclavian and right internal jugular veins. The lymph from the rest of the body including lower extremities; abdomen including liver and intestine; left upper extremity; left side of the chest, head, and neck is drained by the thoracic duct ( Fig. 1 ).The thoracic duct is a long channel, measuring approximately 38 to 45 cm in adults. It typically enters the venous system at the left venous angle formed by the junction of the left subclavian and left internal jugular veins. It starts as a triangular or saccular dilation over the first or second lumbar vertebral body, the cisterna chyli, and runs on the right side of the aorta over the spine. It crosses over to the left side at approximately the fifth thoracic vertebral body in the superior mediastinum and ultimately runs on the left side of the spine. The anatomy of the thoracic duct is variable. Similarly, the cisterna chyli can have variable shape including an inverted Y or V and a string of pearls. The cisterna chyli measures approximately 5 to 20 mm in width and 5 to 7 cm in craniocaudal dimension in adults, and its caliber may change with contraction of the lymphatic pathways. The thoracic duct transports approximately 1.5 to 2.5 L of lymph/chyle daily.

The lymphatic vessels can be divided into 3 types depending on their origin: soft tissue, liver, and intestinal lymphatic ducts. The intestinal lymphatic system absorbs the long-chain lipids from the intestine, reflected by higher concentration of lipids and proteins (60% of the blood level) in the intestinal lymph (also known as chyle). The liver lymphatic system delivers the proteins that are synthesized by the liver into the blood stream, and in fact, liver lymph contains the highest concentration of proteins of all lymph (80%–90% of the blood level), compared with a lower concentration of proteins in the peripheral lymph (17%–30% of the blood level). , Understanding of the biochemical differences of different types of lymph can help in the diagnosis of the origin of the lymphatic leaks by biochemical analysis of composition of the chylous fluid.
Methods of imaging the lymphatic system
Over the years, imaging of the lymphatic system has evolved from direct lymphangiography through cannulation of peripheral lymphatic ducts on the dorsum of feet and hands and interstitial injection of contrast media into interdigital web spaces to injection of contrast media directly into lymph nodes. Modalities of lymphatic imaging include lymphoscintigraphy, fluoroscopic lymphangiography, and, more recently, MR imaging.
Lymphoscintigraphy
Lymphoscintigraphy is performed by injection of radioactive tracers intracutaneously or subcutaneously into the feet or hands. The tracers are rapidly absorbed into terminal lymphatic ducts. Lymphoscintigraphy provides dynamic information but lacks spatial resolution and anatomic details.
Fluoroscopic Peripheral Lymphangiography
Direct lymphangiography is performed through cannulation of peripheral lymphatic ducts on the dorsum of feet or hands, interstitial injection into interdigital web spaces, or cannulation of lymph nodes in the groins and injection of iodized oil-based contrast media (Lipiodol; Guerbet LLC, Bloomington, IN, USA).
Injections directly into peripheral ducts or into the interstitium can be technically challenging and time consuming especially in small children. Furthermore, this peripheral technique may not be adequate to visualize CCL including retroperitoneal lymphatic channels, the cisterna chyli, and the thoracic duct.
Injection of contrast media into the groin lymph nodes for selective visualization of CCL has been used for many decades. Ultrasound-guided intranodal injection of oil-based contrast media with imaging of CCL under fluoroscopy has been refined in children and successfully used for subsequent lymphatic interventions in recent years. , This technique provides excellent spatial resolution, but it is limited by exposure to ionizing radiation, longer examination time than DCMRL, somewhat limited dynamic information due to slow movement of viscous contrast media, and limited information about the relationship of lymphatic ducts with surrounding structures. Importantly, oil-based contrast media poses a potential risk of paradoxic embolization in children with right to left shunts from congenital heart disease or large pulmonary arteriovenous malformations. Cerebral lipiodol embolism has been reported in a child with congenital heart disease and plastic bronchitis (PB) after lymphatic embolization due to direct connection between a lymphatic channel and a pulmonary venous branch.
The limitations of intranodal injection fluoroscopic lymphangiography using lipiodol were overcome by injection of gadolinium-based contrast media (GBCM) into groin lymph nodes in swine models, in conjunction with MR imaging and subsequently used for planning selective lymphatic embolization in children with PB. , , This technique is called DCMRL, which is described later and is the focus of this article.
Peripheral Magnetic Resonance Lymphangiography
Lymphatic ducts in the lower and upper extremities can be imaged by MR imaging using T2-weighted images or with injection of GBCM into the dermal plane in the feet and hands on T1-weighted images. These peripheral MR lymphangiography methods have been used to effectively plan treatments such as lymphovenous bypass in cases of lymphedema. , T2-weighted MR lymphangiography has several limitations including difficulty to visualize smaller lymphatic ducts because of insufficient signal from small amounts of fluid within them and difficulty to differentiate lymphatic ducts from other overlapping fluid-containing structures and veins. Also, it is a static imaging technique that lacks dynamic information, which is important when it comes to demonstrating lymphatic reflux or leakage. Similar to fluoroscopic peripheral lymphangiography, peripheral DCMRL could be technically challenging and time consuming and has not been reported in children yet.
Newer Lymphatic Imaging Approaches
The liver is one of the major producers of lymph fluid. Normally, lymphatic flow is from the liver to the CCL. Hence, the liver lymphatics are not opacified by groin intranodal contrast injection. Until recently, there was no imaging method to visualize liver lymphatics. However, Biko and his colleagues have reported an MR imaging method to image liver lymphatics using T1-weighted MR images and angiographic sequences after injecting GBCM into lymphatics in the liver. In this initial experience, they demonstrated lymphatic flow from liver toward bowel and leakage of contrast into the duodenum in patients with protein losing enteropathy, and leakage into the peritoneal cavity as well as retrograde mesenteric lymphatic perfusion in patients with chylous ascites.
Technique of dynamic intranodal magnetic resonance lymphangiography
After confirming appropriateness of indication, MR compatibility, and normal renal function, ultrasound of the groins is performed to identify good size lymph nodes for cannulation, done in advance of scheduling the DCMRL. This is especially important for infants who may not have sufficiently large lymph nodes for cannulation. Small children undergo the entire procedure under general anesthesia, whereas adolescents and adult patients can undergo the lymph node cannulation under local anesthetic. The following steps are followed in sequence.
Positioning
The patient is placed supine on a detachable MR imaging table, outside the scanning room, with posterior elements of the torso coil underneath the patient before induction of anesthesia or the start of cannulation. Anterior elements of the coil are placed when the patient is taken into the scanner, after the lymph node cannulation.
Lymph Node Cannulation
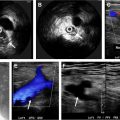
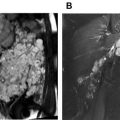
Both inguinal regions are prepped and draped under sterile conditions. Under ultrasound guidance, a 22- to 25-gauge needle is placed in the medulla or central part of an inguinal lymph node on each side. These needles are MR conditional, and their safety can be predetermined on phantoms. Alternatively, angiocatheters can be used but they tend to dislodge frequently. A small volume of saline is gently injected to confirm the needle is appropriately located within the medulla and to ensure there is no extravasation. Injection of saline increases the size of the node ( Fig. 2 ). Some investigators have used contrast-enhanced ultrasound to confirm the needle position in the lymph nodes. The needle is then connected to long 21-inch tubing with a 3-way stopcock on the end and taped in place ( Fig. 3 ). The needle position is again confirmed with saline injection under ultrasound visualization. If the needle is not well positioned or extravasation of normal saline is noted, then the needle is repositioned or another lymph node is cannulated. Syringes with contrast media (dilute gadolinium) and saline flush are connected to the 3-way stopcock (see Fig. 3 ). The patient is then transferred to the MR imaging scanner.


Contrast Media
Any routinely used GBCM can be used for DCMRL. The dose used for DCRML is the same standard dose of 0.1 mmol/kg used for routine intravenous injection. A double dose of 0.2 mmol/kg can be used occasionally in larger patients. The guidelines and precautions used for intravenous injection of GBCM, including assessment of renal function, need to be followed for DCMRL. The total amount of GBCM can be diluted with normal saline 1:1 for older children and adults and 1:2 or 1:3 for younger children to reduce T2 effects of concentrated gadolinium causing darkening from paramagnetic effects of undiluted gadolinium. This practice also increases the volume available for injection, which can be useful in smaller children. Some centers do not dilute the contrast material. The total volume is divided and half of the amount is injected on each side. The entire tubing is primed with contrast containing solution. A syringe with normal saline is also attached to the 3-way stopcock on each side to flush the system and push the contrast remaining in the tube. Contrast media is administered by slow-hand injection and not by pressure injector.
MR Imaging Examination
DCMRL can be performed on 1.5 T or 3 T scanners. , , However, 3 T scanners can potentially provide better visualization of thin lymphatic ducts due to better signal and smaller voxel size achieved at 3 T scanners. Mid-neck to the lesser trochanter is the typical coverage for DCMRL. Some inveatigators use a plastic tray over the abdomen and pelvis to lift the weight of the anterior coil off the patient, to protect the sterile field and minimize dislodgement of the tubing and cannulae.
The 2 main components of MR lymphangiography include T2-weighted imaging and postcontrast dynamic T1-weighted imaging (DCMRL), both acquired with fat suppression. The sequence parameters are listed in Table 1 . As discussed earlier under Peripheral Lymphangiography, T2-weighted lymphatic imaging has several limitations including lack of dynamic information about lymph flow. However, it should be part of the MR lymphangiography for several complementary advantages. T2-weighted images help to localize areas of lymphedema, which may remain undetected by DCMRL. They also provide anatomic information of the cisterna chyli and thoracic duct that can be complementary to DCMRL in cases where the contrast does not propagate into the thoracic duct due to distal obstruction or lymph leak. Furthermore, static, noncontrast T2-weighted MR lymphangiography can be useful in procedural planning for DCMRL and potential intervention. There are no data on frequency of visualization of cisterna chyli and thoracic duct on heavily T2-weighted images. However, on routine T2-weighted images, they are visualized only in approximately 15% of the patients. In our experience with optimized heavily T2-weighted imaging, this demonstrates lymphatic ducts with significantly higher frequency.
| Seq # | Sequence | Plane | TR/TE (ms) | Flip Angle (Degrees) | Matrix/SL | ETL | Pixel Band-Width (Hz) | Approximate Time |
|---|---|---|---|---|---|---|---|---|
| 1 | Single-shot T2 fatsat | Coronal | 2248/160 | 90 | 264 × 230/3 mm | 96 | 399 | 3 min |
| 2 | Single-shot T2 fatsat | Axial | 1376/160 | 90 | 268 × 237/3 mm | 98 | 404 | 4 min |
| 3 | 3D T2 FSE | Coronal | 3168/740 | 180 | 288 × 256/2 mm | 129 | 167 | 4 min |
| 4 | T1W 3D GRE (VIBE/THRIVE/LAVA) | Coronal | 3.5/1.7 | 25 | 308 × 362/2.6 mm | 1 | 574 | 20–30 s |
| GBCM 0.1 mmol/kg, 1:1–1:3 dilution; half dose on each side of groin lymph node hand injected slowly | ||||||||
| 5 | T1W 3D GRE (VIBE/THRIVE/LAVA) every minute till contrast seen at the venous angle | Coronal | 3.5/1.7 | 25 | 308 × 362/2.6 mm | 1 | 574 | 20–30 s |
| 6 | T1W 3D GRE (VIBE/THRIVE/LAVA) as required | Axial | 3.1/1.5 | 25 | 180 × 180/3 mm | 1 | 720 | 20–30 s |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree