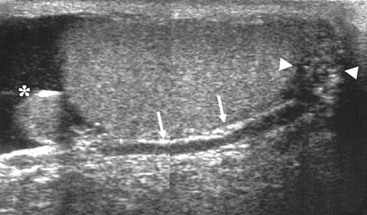
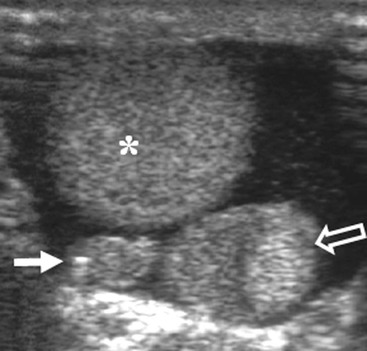
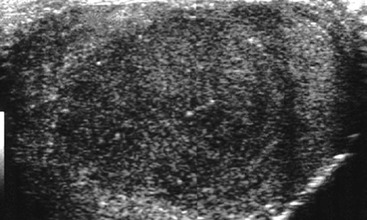
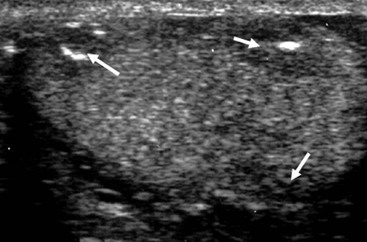
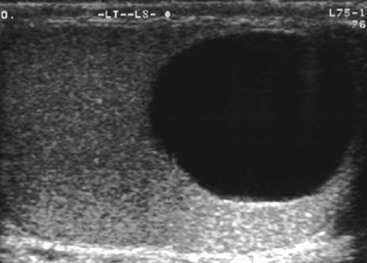
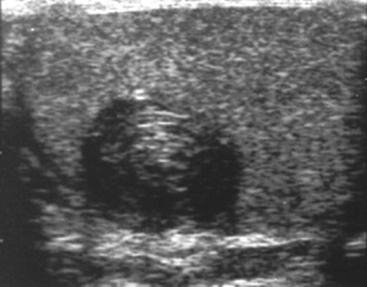
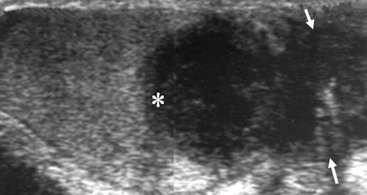
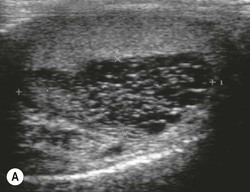
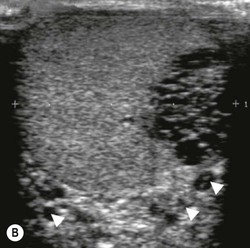
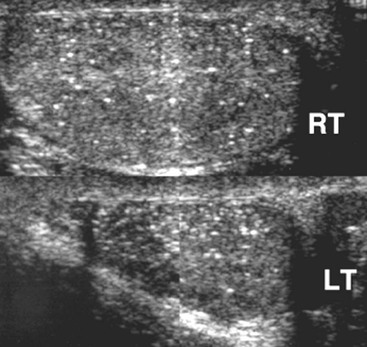
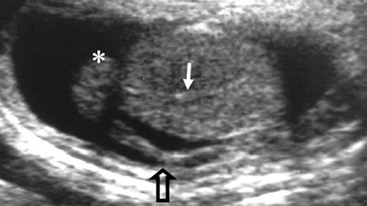
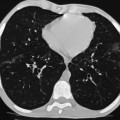
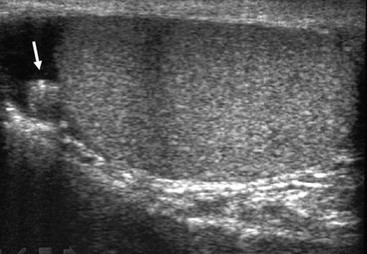
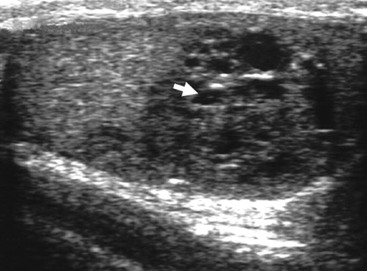
Nadeem Shaida, Lol Berman Although ultrasound (US) is the method of choice for imaging the scrotal contents, an increasing number of publications now describe magnetic resonance imaging (MRI) in the evaluation of testicular masses. MRI has been assessed in patients following an inconclusive US study with a claimed role as a problem-solving tool with particular benefit in differentiating paratesticular from testicular masses and in helping to further characterise masses as solid, cystic or containing fat.1 Computed tomography (CT) and MRI are the investigations of choice in the staging of testicular malignancy and are used in the evaluation of undescended testes that have not been demonstrated sonographically. Radionuclide imaging is still employed in some centres in the evaluation of acute testicular torsion but in practice has now been superseded by colour Doppler US studies. Contrast venography is undertaken to define the anatomy of varicoceles and is often supplemented by embolic techniques, thereby reducing the need for operative intervention in this situation. Intravascular studies with iodinated contrast agents, both venous and arterial, continue to play a part in the assessment of male impotence but Doppler ultrasound may well be able to replace many of these invasive investigations. The testicular parenchyma consists almost entirely of seminiferous tubules enclosed by the fibrous tunica albuginea which extends as septations between lobules of testicular tissue, dividing the testis into several hundred lobules. These septations cannot be imaged except where they converge at the mediastinum testis to produce a linear structure which is reflective on US. The seminiferous tubules merge centrally to form the rete testis, from which efferent tubules perforate the tunica albuginea and convey seminal fluid to the epididymis. The adult testis is 3–5 cm in length and 2–3 cm in transverse diameter and depth. The normal adult testicular volume is 15–20 mL, calculated from the formula: length × width × depth × 0.52 = volume. At the superior pole is a remnant of the Müllerian duct, the appendix of the testis or hydatid of Morgagni, a 3- to 5-mm appendage of similar reflectivity to the testis. A further appendage, the appendix of the epididymis, is situated adjacent to the testicular appendix at the upper pole of the testis (Figs. 40-1–40-3). The epididymis is usually described and illustrated as a tubular structure posterior to the testis. This is far from universal as it may be demonstrated on US laterally or even anterior to the testis. The epididymis comprises a head, tail and body. The head lies adjacent to the superior pole of the testis; the body is formed from the confluence of the ductules of the rete testis to form a single ductus epididymis; this continues as the tail of the epididymis at the inferior aspect of the testis. At the tail of the epididymis, the duct courses cephalad to form the vas deferens. In its developmental descent through the inguinal canal, the testis is invested by a layer of visceral and parietal peritoneum, the processus vaginalis. In normal development the processus obliterates with no residual connection with the peritoneal cavity. The layers of peritoneum surrounding the testis and appendages persist as the tunica vaginalis. The scrotal contents have a triple arterial supply. The deferential artery is a branch of the inferior vesical artery and accompanies the vas deferens, branching into a capillary network at the tail of the epididymis and is the main blood supply to that organ. The testis is predominantly supplied by the testicular artery, which originates directly from the abdominal aorta, inferior to the renal arteries, passing through the inguinal canal with the spermatic cord. The distal testicular artery branches into capsular arteries surrounded by tunica albuginea. Centripetal arteries arise from the capsular arteries, flow towards the mediastinum testis and thence into the testicular parenchyma as arterioles and capillaries. The cremasteric arteries are branches of the inferior epigastric artery, and after accompanying the spermatic cord, anastomose with the testicular and deferential arteries. The venous drainage of the testis is via the pampiniform plexus, draining to the gonadal vein. The scrotal wall and epididymis drain via the cremasteric plexus.2,3 Scrotal masses can be considered as abnormalities arising from the testis and masses arising from adjacent structures. The distinction is frequently obvious to the referring clinician and can usually be made with ease on the ultrasound study with a claimed sensitivity of 100%.4 The specificity for distinguishing malignant from benign intratesticular lesions is less reliable. A false-positive rate of 22% for malignancy was reported in the sonographic evaluation of 23 scrotal masses. The abnormalities contributing to this overdiagnosis of malignancy included testicular infarction, epididymal tumour, organised haematoma and epididymo-orchitis.5 Occasionally, the testicular parenchyma may be compressed and distorted by a large extratesticular lesion to the extent that the distinction between testicular and extratesticular origin is difficult without surgical exploration. Numerous early descriptions of the role of US have advised that intratesticular lesions are to be regarded as malignant until surgically proven to be otherwise.6 This impression has not altered in the intervening years with the proviso that percutaneous biopsy under US guidance may emerge as an alternative to open surgery for defining indeterminate lesions.7 More recent advances in US technology such as the development of sonoelastography to assess the ‘stiffness’ of focal intratesticular lesions have also demonstrated promising early results.8 Testicular malignancies include primary testicular germ cell tumours, non germ cell tumours, metastases, lymphoma and adrenal rest tumours. Primary testicular malignancy comprises approximately 1% of malignancies in men, with an incidence of 3 : 100,000 per annum. This peaks between the age of 15 and 45 years where it accounts from approximately 9% of deaths from malignant disease. The most publicised risk factor is cryptorchidism which appears to be a factor in 10% of primary testicular malignancy. This increased risk persists after orchidopexy and, mysteriously, includes an increased risk of malignancy in the normal contralateral testis in men with unilateral cryptorchidism. Other reported associations include mumps orchitis, testicular microlithiasis, infective orchitis and infertility. The risk of developing a further malignancy in the contralateral testis several years after initial treatment of testicular malignancy is 500–700 times the incidence in the normal population. At present there is no common policy regarding screening any of the at-risk populations although there is an emerging consensus regarding microlithiasis. There is an unexplained rising incidence and geographical variation to testicular malignancy. An incidence of 8 : 100,000 is reported in Denmark compared with 2 : 100,000 in Israel.9 Testicular tumours usually present with a painless unilateral scrotal mass noticed by the patient on self-examination. Approximately 10% present with acute pain thought to be due to haemorrhage. A small group present with metastases from an undeclared primary, which may be demonstrated by US when still clinically impalpable. In this setting the reported negative predictive value of 100% for excluding testicular malignancy is reassuring but should be considered in the light of a recent report of US negative intratubular germ cell neoplasms discovered at orchiectomy in patients with the rare presentation of paraneoplastic meningitis.10 Approximately 95% of primary testicular tumours are of germ cell origin, the remainder arising from Leydig, Sertoli or theca cells that comprise the gonadal stroma. Most germ cell tumours are of single histological type and are seminomas. In order of frequency, other histological types comprise embryonal tumours, yolk sac tumours and teratomas. Approximately 40% of tumours are of mixed cellularity. The peak incidence of seminoma is in the fourth and fifth decades, while the incidence of other germ cell tumours peak approximately a decade earlier. All testicular tumours are rare in childhood, where yolk sac tumours, followed by teratomas, are the most common. Seminomas are generally extremely radiosensitive; they are associated with high levels of alphafeto-protein (AFP) but less often with human chorionic gonadotrophin (hCG). Teratomas respond well to a number of chemotherapeutic agents and are associated with elevated AFP and hCG. They are characteristically less responsive to radiotherapy. The differentiation of seminoma from the other histological types is important clinically but, despite some variation in the ‘textbook’ appearances, there are generally insufficient features to make the distinction using direct imaging or Doppler features. Seminomas are characteristically hyporeflective compared with the surrounding parenchyma and are well-defined and homogeneous (Fig. 40-4). They may also be apparently multifocal on presentation. The typical embryonal cell tumour is less homogeneous and well-defined, while teratomas are of mixed reflectivity and more likely than seminoma to contain cystic spaces and calcifications (Figs. 40-5 and 40-6). Increased tumour flow on Doppler ultrasound is common but not invariable and in practice this cannot be used to differentiate neoplastic from non-neoplastic, benign from malignant or one cell type from another.11 Testicular tumours extend into the surrounding testicular parenchyma and from the mediastinum testis to the epididymis. The lymphatics accompany the venous drainage to retroperitoneal nodes at the level of the renal hila. The local inguinal nodes are not typically involved unless there has been invasion of the scrotal wall. Although up to 96% accuracy has been claimed for ultrasound in the diagnosis of retroperitoneal metastases,12 the widespread availability of more accurate cross-sectional techniques has increased since this report, and in practice ultrasound would no longer be primarily used, although it may still have a role in evaluating visceral metastases. Current best practice suggests the use of CT to assess the retroperitoneal and mediastinal lymph nodes.13 MRI of the retroperitoneum is feasible but as yet has not been widely adopted, due to cost, and instead serves a role in selected patients where repeat CT examinations may be undesirable.14 18-FDG-PET or 18-FDG-PET/CT examinations do not form part of the diagnostic algorithm for initial staging but may be helpful in the evaluation of residual disease after chemotherapy.15 Although a chest radiograph will demonstrate the larger pulmonary and mediastinal metastases, CT reliably demonstrates pulmonary lesions of 3–4 mm in diameter. These are frequently peripheral (subpleural and basal—in line with maximal ventilation and perfusion) but no lung segment is exempt. In the relatively younger age group susceptible to testicular malignancy, pulmonary lesions that may mimic lung metastases such as old granulomata are not common. Nevertheless, care should be taken before assuming that a lung lesion is a metastasis, especially in the absence of mediastinal lymphadenopathy. Mediastinal deposits are less common than pulmonary metastases but care should be taken in the subcarinal region. It is debatable whether iodinated intravenous contrast enhancement should be used routinely. However, close attention should be paid to the mediastinum at soft-tissue settings. Unexpected lesions in the hila or mediastinum with absent tumour markers, favourable histology and a normal retroperitoneum should raise the possibility of coincidental disease such as sarcoidosis. There are few pitfalls in the evaluation of the para-aortic chain apart from the generic problems common to all CT studies of the retroperitoneum such as underfilled bowel loops, anomalous venous anatomy, etc. The spread of testicular malignancy is predictable. Left-sided lesions spread to the upper left para-aortic chain closer to the left renal vein than the aortic bifurcation. Metastases from right-sided lesions tend to be situated slightly more caudad and are usually seen in the anterior interaorticocaval recess. Right-sided lesions may, however, be situated at a more superior level and, when located just posterior to the third part of the duodenum, can prove problematic to the surgeon should resection of a residual mass at this site be required. Only when nodal disease is well established in the ipsilateral side does crossover to the contralateral side and inferior spread to the inguinal nodes occur. Isolated contralateral lymphadenopathy should be viewed with extreme suspicion. Isolated pelvic disease is slightly more common but still rare in uncomplicated testicular tumours. Naturally it can occur where there has been previous testicular maldescent or scrotal involvement. The whole pelvis should be examined at initial staging but it is controversial whether this needs to be undertaken at follow-up in straightforward cases; radiation dose is a consideration in these usually young patients. The accurate staging of testicular malignancy is important and can influence treatment (surveillance, radiotherapy, chemotherapy, etc.). The radiological findings should be considered with other diagnostic features such as cell type and tumour markers. If staging uncertainty persists, a limited repeated CT study at, say, 6 weeks can often resolve the matter. The lung nodule or para-aortic mass may have enlarged and involvement can be assumed. A testicular mass in a patient older than 50 years should raise the suspicion of a metastasis. These are more common in this age group than primary malignancies. Primary sites include the prostate, kidney, bronchus, pancreas bladder and thyroid.6 Although overt clinical involvement is rare in leukaemia, over 50% of men with acute leukaemia and 25% with chronic leukaemia have testicular involvement. The testis is a common site for the occurrence of leukaemia relapse following treatment explained by the theory that there is limited penetration by chemotherapeutic agents at this site. The US features are not specific but are typically of a diffuse multifocal infiltrating lesion which may be of increased reflectivity compared with surrounding testicular parenchyma. Non-Hodgkin’s lymphoma involves the testes in up to 20% of men and may be the ‘primary site’ as well as occurring bilaterally in a proportion of cases. In Hodgkin’s lymphoma, testicular involvement is rare. US patterns of multifocal discrete hypo- as well as hyper-reflective lesions have been described. Cystic lesions, both unicameral and multiseptate, are occasionally encountered, usually as chance findings, and have been reported in up to 10% of testicular ultrasound studies (Fig. 40-7). They are more common in older men.16 Previous trauma, inflammation and embryonal remnants have been described in their aetiology. They should be carefully differentiated from tumours with a cystic component. Epidermoid cysts of the testis have been described varyingly cystic, mixed and solid lesions with a characteristic whorled appearance on US17 (Fig. 40-8). In addition they exhibit no vascular enhancement and appear ‘stiff’ on real-time elastography.18 This has recently been correlated with alternating high- and low-signal layers on T2-weighted MRI images. This is a rare instance where a combination of laboratory tests and imaging findings may suggest that testicle sparing surgery is a possibility.19 Testicular abscess may result from many infective agents and usually demonstrates a low or mixed reflectivity pattern. Although the clinical presentation, often associated with epididymitis, is acute, indolent infections such as tuberculous disease of the testis may have a more insidious onset and be indistinguishable from malignancy (Fig. 40-9). In chronic cases, the presence of calcification within the lesion has been a clue to diagnosis. Percutaneous fine needle aspirate has given the diagnosis without resorting to exploration or orchidectomy where there was clinical suspicion that a lesion was due to tuberculosis.20 This condition is seen in older men and is thought to be due to cystic dilatation of the rete testis. Coexisting epididymal abnormalities, usually consisting of epididymal cysts, have been noted in 85% of cases, leading to the theory that the condition is secondary to obstruction of the epidymis21 (Fig. 40-10). The importance of this finding lies in the recognition of its benign nature. Unnecessary orchiectomy has been undertaken for this entity. In equivocal cases, MRI may be helpful. Characteristic findings have been described as hypointense signal relative to the normal testis on T1 and proton density-weighted sequences, the lesions being undetectable (unlike tumours) and non-enhancing on T2-weighted images.22 The discovery, usually by chance, of multiple sub 1-mm highly reflective foci in one or both testes indicates testicular microlithiasis (Fig. 40-11). The condition is thought to result from degenerating cells in the seminiferous tubules and is currently the subject of much speculation as to its association with testicular malignancy. Microlithiasis has also been reported in association with cryptorchidism, infertility, Klinefelter’s syndrome, testicular infarction, alveolar microlithiasis and numerous other conditions.23 Numerous cases of microlithiasis in association with germ cell tumours have been reported but the exact pre-malignant potential of this condition is unknown. Case reports of malignancy arising in testes under surveillance for this condition are rare.24 The debate over the premalignant potential of this condition continues. A study of asymptomatic Turkish army recruits reported a prevalence of 2.4% in normal adult men, with a mean age of 23 years, and concluded that there is no association with malignancy.25 Conversely, others have concluded that subjects with this condition should be followed up regularly.26 A recent literature review has suggested that clarification to the exact definition of what constitutes microlithiasis would be helpful. The authors also conclude that whilst microlithiasis alone does not require follow-up, in the presence of another risk factor it may be advisable to perform testicular biopsy.27 These lesions arise from many structures within the scrotum and can be considered as cystic or solid lesions. These are far more common than solid lesions. A small amount of fluid surrounding the testicle is a normal finding in most asymptomatic men. A hydrocele may be congenital or acquired and is the commonest cause of scrotal enlargement. Hydroceles also occur in infancy as a result of a patent processus vaginalis and usually resolve spontaneously (Fig. 40-12). On US, hydroceles characteristically appear as fluid collections surrounding one or both testes and are either non-reflective or may contain swirling low-reflectivity echoes due to cholesterol crystals (Fig. 40-13). The fluid surrounds the anterior and lateral aspects of the testis. Hydroceles are usually obvious clinically. Imaging is requested when their size prevents adequate examination of the testis and there is a query about underlying malignancy, a rare association. Multiloculated hydroceles are recognised and may be indistinguishable from an organising haematoma (Fig. 40-14).
Male Genitourinary Tract
Methods of Examination
Anatomy
Scrotal Masses
Testicular Masses
Malignant Testicular Pathology
Staging Testicular Malignancy
Computed Tomography
Non-Primary Testicular Malignancies, Lymphoma and Leukaemia
Non-Malignant Focal Testicular Lesions
Non-Focal Testicular Abnormalities
Tubular Ectasia
Testicular Microlithiasis
Extratesticular Scrotal Lesions
Hypoechoic Lesions

Male Genitourinary Tract
Chapter 40