Primary airway tumors
Metastases
Primary tracheal carcinoma
Bronchogenic
Adenoid cystic carcinoma
Renal cell carcinoma
Carcinoid
Metastatic melanoma
Mucoepidermoid tumor
Breast
Chondrosarcoma
Thyroid
Bronchogenic carcinomas
Prostate
Esophageal cancer
Sarcoma
Thyroid cancer
Lymphoma
Growth of tumors adjacent to the central airways or as a result of mediastinal lymphadenopathy may also result in obstruction due to simple mass effect. Esophageal cancers may extrinsically compress and in some cases erode into the trachea or main stem bronchi (most often the left main stem bronchus) causing a malignant fistula. Thyroid cancers can also result in significant compression of the proximal airways, most often at the level of the extra-thoracic trachea. Lymphomas and other primary tumors that metastasize to the lungs may cause significant extrinsic compression at the level of the lower paratracheal or subcarinal lymph nodes leading to marked reduction in the luminal diameter of the distal trachea and/or main stem bronchi. Figure 25.1 provides a graphic representation of the tumor-airway spatial relationships potentially resulting in malignant central airway obstruction.
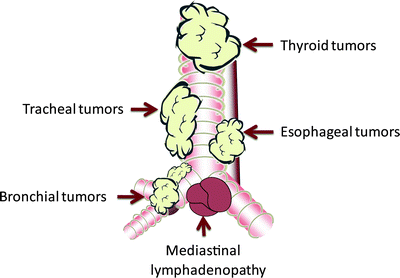

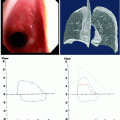
Fig. 25.1
Tumor-airway spatial relationships. The location of the airway mass may be suggestive of its etiology. The figure is a graph representation of typical locations of airway tumor involvement
Classification
Central airway obstruction is generally classified into three categories depending on whether the tumor is purely endoluminal, extraluminal, or mixed. If the tumor is confined to the airway lumen (endoluminal), it is considered “intrinsic” compression. On the other hand, if the tumor is obstructing the airway due to mass effect and there is no endoluminal component, it is called “extrinsic” compression (extraluminal). The majority of central airway obstruction falls into the final “mixed” category, being that there are elements of both intrinsic and extrinsic involvement. With respect to the “mixed” category, the tumor often originates adjacent to the airway and erodes through the airway wall invading the lumen. This classification is quite important as it may impact on the therapeutic approach as discussed later in this chapter. Refer to Fig. 25.2 for a graphic representation of the three categories of airway tumor involvement.
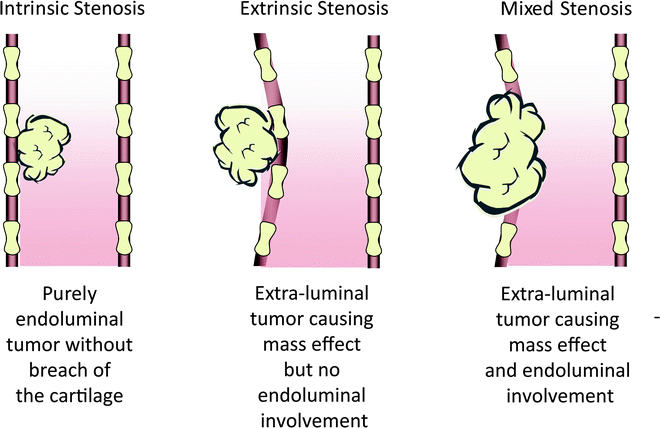

Fig. 25.2
Classification of airway tumor involvement. Tumor involvement in the airway is classified as intrinsic, extrinsic, or mixed based on whether the tumor is limited to the airway lumen, extra-luminal, or a combination of endoluminal and extra-luminal involvement, respectively
Diagnostic Approach
The diagnosis of malignant airway involvement may be delayed as the signs and symptoms can be quite nonspecific. These patients have frequently benefited from several courses of antibiotics prior to evaluation. Clinicians should have a low threshold to consider malignancy in high-risk patients, i.e., those with a prior or current diagnosis, strong family history of cancer, and exposure to known carcinogens such as tobacco or radiation. A comprehensive history and physical examination is necessary to determine the most appropriate diagnostic and staging evaluation. The clinical findings can also establish the urgency to intervene as well as provide information regarding special precautions necessary during the procedure.
With respect to diagnostic tests, a chest x-ray is often available; however, its utility in the management of these patients may be quite limited. Often, the airways are poorly visualized and this imaging technique provides scant information regarding the extent of the disease. The major value of the plain radiograph lies in its ability to provide information regarding the natural history of the tumor. In the case of atelectasis/lung collapse downstream from an obstruction, a longer duration of atelectasis renders the patient less likely to benefit from an airway intervention. Prior radiographs may provide some insight into the time course of the disease and likelihood of response to intervention but will add little to the actual treatment planning.
On the other hand, computed tomography (CT) can be quite helpful in that it may provide information regarding invasion by the tumor into the airways and mediastinal structures. The orientation of vascular structures as well as their patency also can provide valuable information for treatment planning. A CT scan, particularly with three-dimensional reconstructions, may help with decisions regarding selection of debridement technique and/or prostheses such as stents.
The ability to perform functional testing depends on the initial presentation. Spirometry may provide valuable information regarding both the severity and location, albeit intrathoracic or extra-thoracic. The spirometric abnormalities fall into three categories: (1) fixed airway obstruction with reductions of both inspiratory and expiratory flow rates and a box-like shape on the flow-volume loop, (2) variable intrathoracic obstruction with flow limitation on expiration and a reduction in the effort-dependent portion of the expiratory loop, and (3) variable extra-thoracic that shows reduction in the effort-dependent portion of the inspiratory limb of the flow-volume loop resulting from an inspiratory flow limitation. Despite concerns regarding the stability of these patients, Tremblay and colleagues recently showed that bedside spirometry may be safely performed in the majority of these patients and that marked post-procedure changes could be demonstrated which correlate with improvements in measures of quality of life.
Rigid Versus Flexible Bronchoscopy
The gold standard of diagnostic testing for malignant airway obstruction is bronchoscopy. The safety and efficacy of rigid versus flexible bronchoscopy as an initial tool remains controversial. The risks of flexible bronchoscopy without the ability to perform rigid bronchoscopy are many. Certainly in an already compromised airway, the major concern is that of destabilizing the already compensating patient by further limiting airflow. The bronchoscope itself may further limit the lumen and any airway trauma result in bleeding or edema aggravating the situation. A standard adult therapeutic bronchoscope may significantly reduce the ability to ventilate even through an endotracheal tube depending on the caliber of the tube. Another aspect to consider when selecting either flexible versus rigid bronchoscopy in the initial diagnostic approach is that of moderate sedation. Anesthesia for rigid bronchoscopy will be addressed separately. Commonly employed agents for moderate sedation include benzodiazepines and narcotics such as fentanyl. These sedatives may depress ventilation and relax respiratory musculature both of which may compromise the airway to a greater degree.
There are many advantages to the rigid bronchoscope in this setting are many. The major advantage is the ability to ventilate the patient while reestablishing airway patency. The rigid bronchoscope may also be used to tamponade any bleeding, selectively intubate one of the main stem bronchi, and/or mechanically debride the airway. The flexible bronchoscope may be used through the rigid barrel allowing for the evaluation of airways distal to the obstruction, particularly if using a smaller borescope such as the ultrathin bronchoscope. The flexible scope may be used to classify the lesion as intrinsic, extrinsic, or mixed obstruction; determine whether there are indeed airways beyond the obstruction; and measure the length of lesion. Unfortunately, the utility of the flexible bronchoscope to perform pulmonary toilet in the case of inspissated secretions distal to an obstruction may be limited by the caliber of the working channel.
Initial Airway Stabilization
The airway often requires initial stabilization as a bridge to definitive management. A very simple maneuver is to ensure optimal positioning of the patient to enhance pulmonary mechanics. Patients should be seated, rather than in the supine position. This may help on two fronts: first of all, it may help relieve any additional mass effect on the trachea, and secondly it may render respiratory muscle function more efficient. Supplemental oxygen should be applied via nasal cannulae or mask; however, often oxygenation does not appear to be problematic. Most frequently the major issue is that of turbulent airflow resulting from the obstruction. Turbulent flow is less efficient than laminar flow and may manifest clinically as increased work during breathing. Helium’s density is approximately 1/3 that of oxygen, resulting in a reduction in Reynold’s number and an increased tendency for more laminar flow. Laminar flow produces less resistance than turbulent flow, and therefore, there is more flow for a given driving pressure. In order to optimize the flow characteristics, combinations of helium(70–80 %) and oxygen (20–30 %), called Heliox, may be administered. In patients requiring a higher fraction of oxygen, Heliox may not be appropriate. Patients with significant airway obstruction frequently have little reserve, and therefore, it is very important not to delay optimizing the oxygenation and airflow characteristics until the patient desaturates or deteriorates clinically.
Patient Selection for Airway Interventions
No literature exists regarding patient selection for airway interventions. In practice, most would agree that the patient should be symptomatic and that the symptoms are attributable to the airway obstruction. In select cases, airway interventions are considered prior to the onset of symptoms, especially if the procedure is also being performed for diagnostic or staging purposes. Endoscopic evaluation of the airway can help with staging and disease extent is often much more reliably determined based on bronchoscopic evaluation as compared to imaging. Endoscopic staging techniques include simple white light imaging to determine the tumor origin and proximity or involvement of the main carina. In addition, white light bronchoscopy may reveal previously undetected contralateral tumor involvement. Adjuncts to white light bronchoscopy that may add to the sensitivity to detect disease extent include autofluorescence bronchoscopy, narrowband imaging, or alternatively endobronchial ultrasound. Both autofluorescence bronchoscopy and narrowband imaging are techniques whereby wavelengths of light are utilized to determine the characteristics of the tissue. Detection of increased vascularity or changes in absorption/reflection characteristics may be suggestive of disease extension beyond the limits of the endoscopic abnormalities detected by white light bronchoscopy. With respect to endobronchial ultrasound, it may be useful in some cases to determine whether or not there is invasion of the airway. The endobronchial ultrasound may demonstrate that the tumor is confined to the lumen of the airway and is not invading through the cartilage. In this case, the patient may be amenable to endoscopic curative treatment particularly if a poor surgical candidate. Alternatively, the tumor may be completely outside of the airway and simply pushing on the airway. This again may influence the surgeons with respect to their surgical approach. In the case of esophageal or thyroid tumors, the fact that the tumor does not invade the airway but rather compresses may obviate the need for tracheal resection.
Evidence of distal airway patency and/or blood flow is preferable as there is a risk of worsening ventilation-perfusion matching if airway patency is reestablished despite lack of regional blood flow. This may be difficult to determine as there may be hypoxic vasoconstriction, which resolves once the airway is patent. Standard diagnostic tests such as ventilation-perfusion scans fail to identify patients at a higher probability of benefiting from airway intervention. A vascular cutoff sign may suggest a worse outcome of airway tumor debulking. If there is radiographic evidence of atelectasis/collapse distal to the obstruction that has been documented for a protracted period, i.e., several months, there is a lower likelihood of lung re-expansion post-procedure. Although there is no set point beyond which intervention should not be considered, the lung being down for a period of less than 30 days seems to portend a better outcome. If the patient is a candidate for curative intent management in a timely fashion, it may be best to limit interventions to those with potential to improve his/her chance of response to treatment. Examples could include debridement to determine tumor extent and operability or to allow for drainage in post-obstructive pneumonia prior to initiation of chemotherapy and/or radiation. Interestingly, patients initially thought not to be candidates for curative intent therapy may be found to actually be candidates for surgery. For example, tumors emanating from the upper lobes have a propensity to grow into and obstruct the main bronchi and bronchus intermedius or lower lobe. Following endoscopic resection, the tumor origin may be identified, and the bronchoscopist determines whether the tumor is confined to the upper lobe or extends into other lobes. The final consideration is that of life expectancy. Patients destined not to survive for a period sufficient to gain benefit from the intervention should be deferred. Just because you are technically able to open an airway does not necessarily mean it will benefit the patient, and in fact, it is essential to balance the potential risks and benefits of intervention of every case.
Anesthesia for Endoscopic Management of Malignant Airway Obstruction
Anesthesia for rigid bronchoscopy requires close collaboration and effective communication between the endoscopist and anesthetist. The majority of these procedures are performed in patients with comorbidities who are considered at high risk of adverse outcomes but for whom alternative less risky options do not exist. Most of these patients are classified by the American Society of Anesthesiologists (ASA) physical status classification of III-IV. In other words, they have severe systemic disease that may be a risk to life. In addition, many of these cases have a modifier to account for the prognostic impact of the urgency of the case.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree