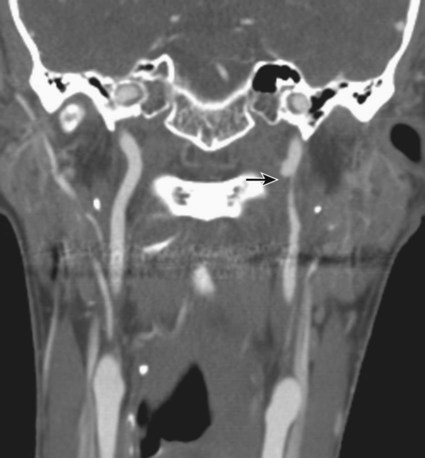
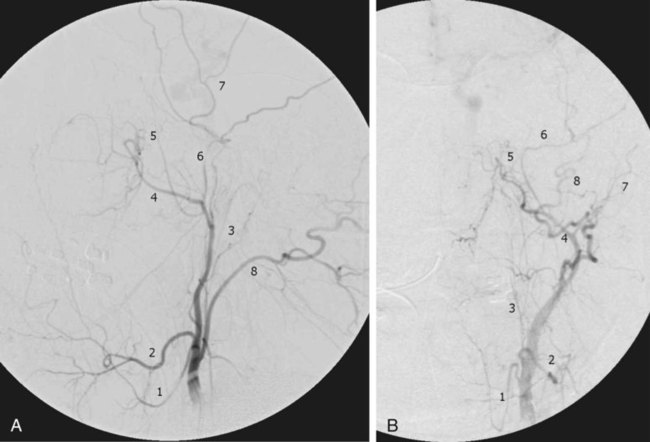
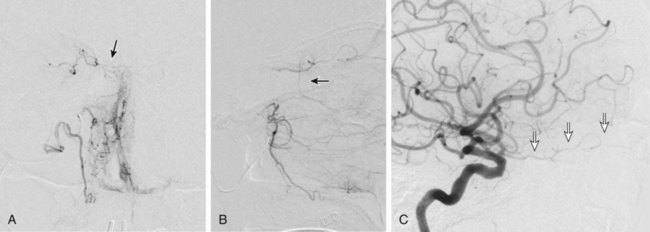
Traumatic head and neck vascular injuries such as dissections, transections, pseudoaneurysms, arteriovenous fistulas, and large artery occlusions are relatively uncommon but can result in potentially devastating stroke, severe blood loss, and even exsanguination and death. Rapid, early diagnostic imaging followed by effective management of neurovascular injuries can help improve patient outcome by focusing attention on prompt treatment of these lesions.1,2 Advances in multidetector computed tomography (CT) technology (Fig. 97-1) have greatly facilitated rapid noninvasive screening of trauma patients with suspected neurovascular injury. Although the use of computed tomographic angiography (CTA) for definitive diagnosis remains somewhat controversial, it can provide sufficient information to “fast-track” a patient with significant vascular injury to definitive surgical or endovascular intervention.3 Alternatively, CTA may help determine whether a patient with minor injury is better served by delaying angiographic evaluation or treatment in favor of managing concomitant visceral or orthopedic injuries. Angiographic and endovascular resources can thus be directed where they are most appropriate and most needed. • Obvious signs of trauma, including facial fracture; penetrating wounds; epistaxis; hemorrhage from the mouth, orbit, or ears; periorbital ecchymosis, or Battle sign; expanding cervical hematoma or a pulsatile mass, cervical bruising or abrasions; or cervical bruit in a young patient1,2 • Fractures in proximity to the internal carotid or vertebral arteries, such as basilar skull fractures, cervical spine fracture-dislocations, or fractures extending into the foramen transversarium1,2 • History of significant blunt force injury to the head and neck, especially with a mechanism of injury involving cervical hyperextension-rotation, hyperflexion, or direct blow to the neck • New focal neurologic deficit or stroke of unexplained cause on head CT in a patient with history of trauma; focal neurologic deficit in the setting of questionable or minimal trauma, especially if the patient was normal at admission1 • Pain in the neck, face, scalp, or head in the setting of questionable or minor trauma Relative contraindications include: • Presence of dangerous collaterals or routes of blood flow to the orbit and brain; if these are present and cannot be protected, endovascular therapy may pose a significant risk for stroke. • Reopening of an acute traumatic vascular occlusion should be done with extreme caution because of the risk of thromboembolic stroke, and should be performed only when essential for cerebral perfusion. In many instances, particularly in patients with an intact circle of Willis and good intracranial collaterals, intentional occlusion of severely injured vessels or coil embolization to secure a traumatic vascular occlusion should be considered among the treatment options. • Some patients may better be served by medical or surgical therapy. • Triage issues may dictate the timing or extent of treatment. • Continuous flush system using heparinized saline (2000-4000 units/L) for sheaths, catheters, and microcatheters) (Fig. e97-1) • 6F (or larger) access sheath • 6F (or larger) introducer guide sheath, 90 to 100 cm long, for stent delivery • End-hole microcatheter appropriate for use with chosen embolic agents, and 0.014-inch microwire; exchange length microwire may be needed for some stent delivery systems or tortuous anatomy • Particulate embolic agents for epistaxis and facial hemorrhage • Liquid embolic agents (n-butyl cyanoacrylate [NBCA], Onyx) have been used for treatment of life-threatening facial hemorrhage. • Fibered 0.018-inch push coils and coil pusher for distal arterial injuries • Fibered 0.035-inch push coils and Benson 0.035-inch wire for proximal arterial injuries • Detachable 0.020-inch, 0.018-inch, 0.014-inch, 0.012-inch coils for occlusion of pseudoaneurysm sacs • Stent(s) suitable for delivery into the extracranial internal carotid or vertebral arteries for treatment of cervical arterial dissection or pseudoaneurysm • Covered stent(s) suitable for delivery into the extracranial internal carotid artery for treatment of cervical arterial lacerations and transections, including arteriovenous and cavernous carotid fistulae (CCF) The cervical, facial, and cranial regions are supplied by a rich vascular tree, including the external carotid artery (ECA), internal carotid artery (ICA), vertebral artery (VA), thyrocervical trunk, and costocervical trunk. Branching patterns can be variable, and numerous potential pathways of collateral flow exist between the extracranial and intracranial arteries.4 Thus, careful vigilance for the presence of these dangerous collaterals is essential in preventing inadvertent embolization of the retina, brain, or cranial nerves. If time and the patient’s condition permit, CTA to evaluate the extent of bony injury and identify potential sites of arterial injury often facilitates delivery of definitive treatment, and is well worth the additional “cost” in examination time, contrast administration, and patient radiation exposure.3 In epistaxis and facial smash injuries, the most common culprits amenable to embolization are branches of the facial and internal maxillary arteries. The facial artery arises as a proximal branch of the ECA (either directly or as a common trunk with the lingual artery), ascends superficial to the mandible, then courses medially in the superficial soft tissues of the face, giving off branches to the lips, mandible, cheeks, and nasal cavity. The internal maxillary artery (IMA) arises as one of the terminal branches of the ECA at the level of the ramus of the mandible. It passes forward almost horizontally, medial to the mandibular ramus, giving off branches to the soft tissues (transverse facial artery), meninges (middle and accessory meningeal arteries) and, as it subsequently courses either deep or superficial to the lateral pterygoid muscle, to the muscles of mastication (masseter, temporalis, mylohyoid, and pterygoids). The most distal part of the IMA passes through the pterygomaxillary fissure and into the pterygopalatine fossa; this distal segment gives rise to numerous branches that can be injured in facial fractures, particularly LeFort fractures, including the anterior and posterior superior alveolar arteries, descending palatine artery, infraorbital artery, sphenopalatine artery, and branches to the nasal cavity (Fig. 97-2). Facial and nasal cavity bleeding can also occur as a consequence of injury to intracranial branches of the ICA. Persistent nasal bleeding may arise from the posterior and anterior ethmoidal arteries, which arise from the ophthalmic artery (OphA) distal to the takeoff of the central retinal artery. The posterior ethmoidal artery courses medially, passing between the medial rectus and superior oblique muscles, and enters the nasal cavity via the posterior ethmoidal canal to supply posterior nasal air cells and the upper nasal septum. The anterior ethmoidal artery also courses medially, passing through the anterior ethmoidal canal, and supplies the anterior and middle ethmoidal air cells, frontal sinus, and anterosuperior aspect of the lateral nasal wall. These ethmoidal branches are generally not amenable to safe direct embolization, and a failure to recognize them prior to ECA branch embolization can result in unilateral vision loss or stroke (Fig. 97-3).
Management of Head and Neck Injuries
Indications
Contraindications
Equipment
![]()
Technique: Epistaxis, Facial Fractures
Anatomy and Approaches
Management of Head and Neck Injuries