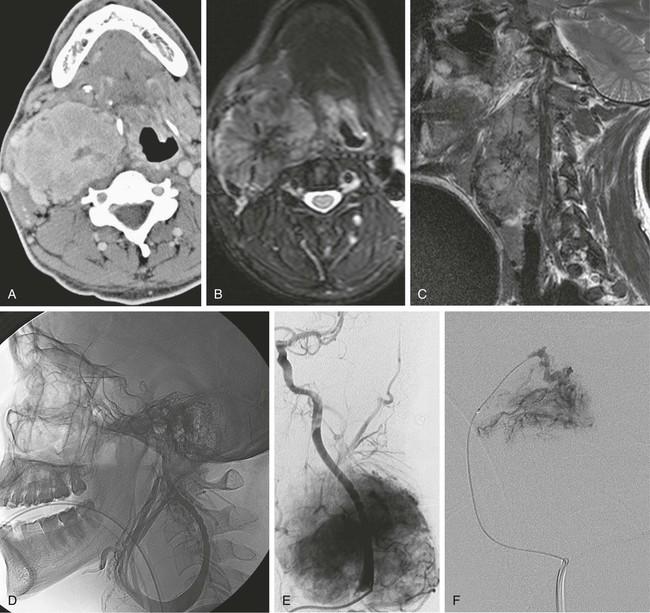
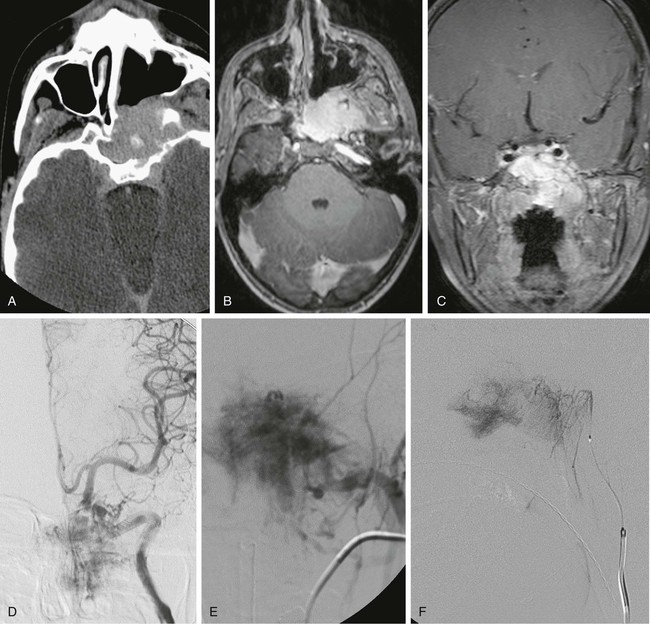
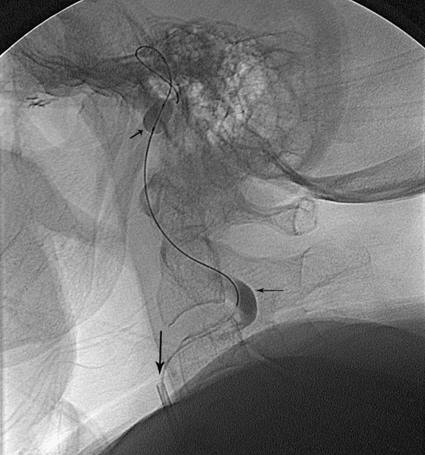
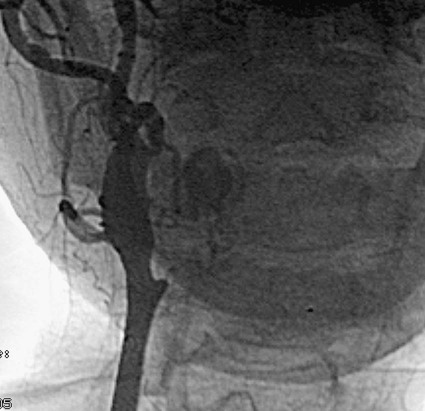
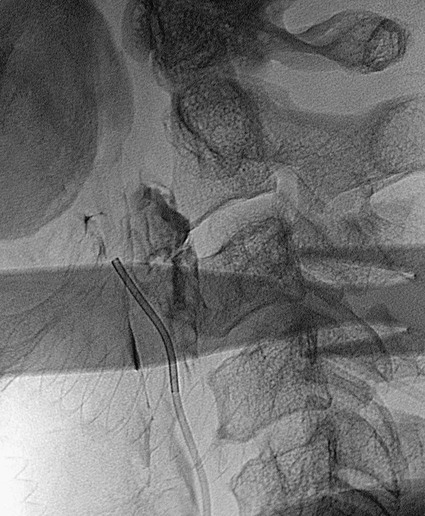
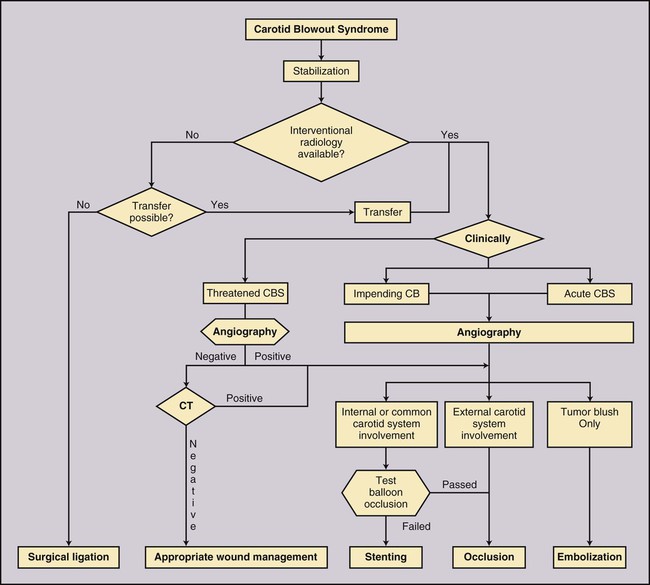
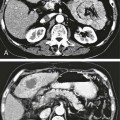
Todd S. Miller, Elizabeth R. Tang, Randall P. Owen, Jacqueline A. Bello and Allan L. Brook The types of masses encountered in the head and neck are diverse, as are their appropriate treatments. Some benign head and neck lesions are treated primarily by surgical means without endovascular intervention. For example, when possible, hemangiomas are commonly excised by laser, and lymphangiomas are sclerosed by direct puncture or surgically resected.1 Certain highly vascular benign tumors like carotid body paragangliomas and juvenile nasal angiofibromas are frequently treated with preoperative embolization. Malignancy is treated by surgery whenever possible.2,3 Often, however, these patients present with the tumor at a high stage. For stage IV squamous cell carcinoma, 5-year survival rates are lower than 25% following surgery and/or radiotherapy.4 Management of these serious late-stage malignancies is complex, involving substantial teamwork among several disciplines. The interventional radiologist’s role on this team is most necessary for advanced tumors or carotid blowout syndrome (CBS) secondary to such tumors and/or complications of treatment. Potential interventions include balloon test occlusion (BTO), preoperative embolization to aid surgery or posttreatment embolization for hemorrhage due to tumor necrosis, emergent treatment of CBS, intraarterial chemotherapy, and image-guided radiofrequency ablation (RFA).5 The two most common endovascularly treated benign lesions of the head and neck are paragangliomas and juvenile nasal angiofibromas (JNAs). Preoperative embolization of other potentially highly vascular head/neck tumors, including angiomyomas (i.e., angioleiomyoma, vascular leiomyoma),6,7 neurofibromas,8 schwannomas,9,10 hemangioendothelioma,11 and meningioma1 has been reported. Congenital vascular malformations that have failed to regress or those whose mass effect impinges on vital structures have also been successfully treated with endovascular therapy.12 Paragangliomas are slow-growing tumors that originate from the neural crest of the autonomic nervous system and can be hereditary in 7% to 9% of cases.13 Locations in the head and neck include the carotid body (35%), glomus vagale (11%), tympanic and/or jugular regions (almost 50%; jugulotympanic if the tumor is larger and melds together over the two regions), and other locations (6%) including the orbit, nasal cavity, and the rare laryngeal case, 90% of which are supraglottic.14 The malignant potential of paragangliomas is generally low at around 4%,15 but it can be up to 10% to 18% for the vagal, carotid, and laryngeal types.3 Recommended therapy for paragangliomas is complete surgical excision. Given the high vascularity of these tumors, the role of endovascular management by preoperative angiogram and embolization can be significant. If a paraganglioma is suspected, a diagnostic superselective angiogram should precede any biopsy attempt, lest the attempt lead to arterial rupture. The angiogram should include bilateral exploration,16 since multicentric paragangliomas occur in up to 80% of familial cases and 10% to 20% of nonfamilial,17 and bilateral carotid body tumors occur in 5% of sporadic and 33% to 38% of familial lesions.13 In addition, diagnostic angiography may demonstrate occult lesions not previously demonstrated on computed tomography (CT) or magnetic resonance imaging (MRI).16 Diagnostic evaluation demonstrates the blood supply and flow dynamics of the tumor. The venous supply can be mapped, allowing the surgeon to preserve the venous drainage, limiting blood loss. The role of embolization in paraganglioma management depends on the extent of the tumor and the anatomy of its feeding branches.3,16,17 In jugular or jugulotympanic paragangliomas with more complicated vasculature involvement, preoperative embolization has become a definite part of surgical management and can significantly reduce intraoperative blood loss.17,18 In most tympanic and laryngeal cases, preoperative embolization is unnecessary.17,19 Transarterial or direct puncture embolization as an alternative to radiotherapy could be considered as the primary therapy for paragangliomas that are unresectable or where resection would be debilitating.1,20–22,63 As for the management of carotid body and vagal paragangliomas, most recommend preoperative embolization, but the role of preoperative embolization is still a matter of debate, especially it seems for carotid body tumors.14,17,23 Carotid body tumors are potentially the most challenging to resect. This tumor originates in the bifurcation of the common carotid artery (CCA), where it commonly involves the internal carotid artery (ICA) and receives feeding vessels from external carotid branches and muscular branches of the ICA.24 When small, hemostasis can be controlled during resection through careful preoperative and intraoperative consideration of the ascending pharyngeal artery (Fig. 94-1).14,13,11 Finally, if resection of the paraganglioma may require sacrifice of the carotid artery, particularly in the case of large jugulotympanic tumors and larger carotid body tumors, another necessary preoperative endovascular intervention is BTO of the ICA.24 JNAs are benign, submucosal, unencapsulated, vascular tumors that are most commonly diagnosed in adolescent males between 14 and 25 years old, but can present at any age. The tumor is locally destructive and tends to erode bone as it spreads.24 Patients usually present with unilateral nasal obstruction or epistaxis. The majority originate in the sphenopalatine foramen and progress to the pterygopalatine fossa, infratemporal fossa, sphenoid sinus, and/or other locations within the head/neck.25,26 Though benign, the risk of bleeding and/or further intracranial extension can be life threatening.27 Surgical resection is considered one of the best treatment options, in which preoperative embolization plays a significant role to prevent excessive intraoperative blood loss.28 Determining the extent of intracranial involvement is crucial to treatment planning for these lesions, as is determining the vascular supply, which may include ipsi- and contralateral, internal and external carotid artery (ECA) systems.29 Pretherapeutic planning often involves endoscopic biopsy, CT scan, MRI, and diagnostic angiography (Fig. 94-2). In cases where diagnostic angiography shows risk of surgical injury to or potential involvement of the ICA, BTO of the ICA should be used to determine adequacy of collateral blood flow. Because profuse intraoperative bleeding is a hallmark of JNA surgery, preoperative embolization of feeding vessels from the external carotid system is consistently recommended in the ear, nose, and throat literature, although some authors rely on intraoperative ligation of feeding vessels.25,29,30,31 Decreased blood loss, surgical time and morbidity have been demonstrated with either approach. This consideration is crucial because the relatively lower intravascular blood volume of children limits their tolerance for blood loss. Direct embolization with Onyx has been performed as part of endoscopic resection as well.32 Congenital vascular malformations are primarily treated with percutaneous therapy, where direct puncture of these lesions allows instillation of sclerosing agents. In some cases, however, endovascular techniques can assist in therapy (Fig. 94-3). Congenital vascular malformations are categorized as either low- or high-flow lesions. The low-flow group includes telangiectasias, lymphangiomas, and hemangiomas. Although sclerotherapy is the dominant therapy for this group,12,33 when hemangiomas present as superficial fungating hemorrhagic lesions large enough to sequester platelets or obstruct vision or vital structures, polyvinyl alcohol (PVA) embolization may be used conservatively.3,34 To prevent disfigurement or facial nerve injury when hemangiomas present in the face, surgical excision is usually performed but requires complete resection to minimize the high rate of recurrence. Preoperative embolization assists resection by shrinking the tumor and also helps preserve cosmetic appearance following surgery.1,35,36 The high-flow group, in addition to JNAs, includes arteriovenous fistulas (AVFs)/aneurysms and arteriovenous malformations (AVMs). Kohout et al.’s study (1998), the largest analysis of patients with head/neck AVMs, showed the most success with those lesions treated by a combination of super-selective embolization and aggressive surgical resection.37,38 Although embolization and surgery lead to a much higher curative rate for head/neck AVMs, the complication rate has been reported as almost double that of ethanol sclerotherapy.39 Thus, Jeong emphasized that ethanol sclerotherapy should be considered the primary treatment modality, followed by a combination of embolization and surgery if repeated sclerotherapy fails.39 Surgical resection maintains an important role in the management of head/neck carcinomas; indeed, complete resection of head/neck malignancy with negative histologic margins is the most crucial factor in prognosis.2 Even in cases of residual or recurrent advanced head/neck carcinoma involving the carotid artery, where prognosis continues to be dim despite resection, surgery can reduce the incidence of life-threatening sequelae like fistula formation, erosion of the pharyngeal wall, or acute tumoral hemorrhage.40,41 Most head/neck malignancies are relatively hypovascular and do not require preoperative embolization for surgical resection,42 but involvement of the carotid artery by head and neck carcinoma can hinder safe tumor resection. In these cases, preoperative BTO followed by preoperative carotid artery occlusion can greatly reduce neurologic morbidity and assist in planning for resection of tumor involving the carotid artery. Candidates for surgery with preoperative embolization include those whose carotid arteries are invaded by the less radiosensitive carcinomas (e.g., adenocarcinoma, adenoid cystic carcinoma, melanoma) or any residual or recurrent head/neck carcinomas including squamous cell.43 Usually such a patient will present with a fixed neck mass. Clinical presentation, however, has a highly inaccurate (32%-63%) rate of predicting carotid invasion. Circumferential carotid artery involvement of at least 270 degrees should be confirmed by MRI, CT, or ultrasound, which predicts arterial wall invasion with high sensitivity and specificity. In one series, arteriography only identified invasion in 1 of 12 patients2,44,45 (Fig. 94-4). Historically, carotid artery ligation can cause up to 45% neurologic morbidity rate and 31% to 41% reported mortality.2 Stroke can occur either immediately from decreased blood flow or later from a postprocedural thrombus or embolic event.43 Preoperative BTO with/without single-photon emission computed tomography (SPECT) imaging or induced hypotension can help identify those who would be less neurologically vulnerable to decreased blood flow. Patients whose preocclusion diagnostic evaluation indicates inadequate collateral flow may benefit from a bypass procedure during surgical ligation, though this approach adds morbidity and mortality. For those patients with demonstrated sufficient collateral flow allowing for carotid resection without reconstruction, preoperative coil embolization of the ICA can minimize surgical blood loss as well as minimize postprocedural thromboembolic complications. In particular, coil embolization of the preophthalmic petrous carotid artery with postembolization heparinization has been shown to help prevent distal embolization after surgical resection.43 After allowing clot formation to stabilize, surgical ligation can follow 2 to 6 weeks later42 (Fig. 94-5). Rupture of any branch in the extracranial carotid artery system, known as carotid blowout, can follow extravasation, pseudoaneurysm, or AVF from any number of causes and can lead to acute transoral or transcervical hemorrhage. Carotid blowout syndrome is a broader term that encompasses carotid blowout as well as the threat of it in cases where the carotid is injured or exposed.46 Most commonly, the cause leading to carotid blowout is therapy for squamous cell carcinoma (Figs. 94-6 and 94-7). Radiation weakens the arterial wall and has been associated with a sevenfold increase in the risk of CBS. Other etiologies include other tumors, neck trauma, and functional endoscopic surgery for refractory epistaxis.47 Historically, treatment of CBS with emergent surgical ligation alone led to a 60% neurologic complication rate and 40% overall mortality.48,49 With advances in interventional endovascular therapies that include embolization, occlusion, and stenting, morbidity and mortality has been reduced substantially. Attempts should therefore be made to treat CBS endovascularly. A combination of anatomy, severity, and availability of interventional services determines the appropriate endovascular management of CBS. A helpful algorithm has been proposed by Cohen and Rad47 (Fig. 94-8). If CBS severity falls under the categories of threatened by pseudoaneurysm or neoplasm, or impending or acute, angiography and CT with contrast should be used to confirm the anatomy and the potential of sentinel hemorrhage.50 If ICA or CCA involvement is demonstrated by angiography/CT, BTO is indicated to determine extent of collateral flow. If collateral flow is inadequate, stenting is indicated to achieve hemostasis until permanent occlusion with surgical extra-anatomic bypass (i.e., subclavian to carotid) can be performed. Covered stent technologies have advanced to a point where their use in such patients may also be beneficial.51 ICA/CCA involvement with adequate collateral flow as well as ECA involvement should be managed by distal and proximal occlusion using coils or detachable balloons (currently unavailable in the United States). If the tumor itself is the source of hemorrhage, as indicated by tumor blush on angiography/CT, the entire tumor bed should be embolized. This can be accomplished with PVA particles via superselective transarterial microcatheterization of all arterial supply to the tumor or by direct-puncture ethanol or cyanoacrylate as a last resort48 (Table 94-1). TABLE 94-1 Balloon Test Occlusion Decision Matrix*: Indications for Bypass from Temporary Balloon Occlusion: Institutional Protocol *The suggested testing matrix was devised from multiple components of balloon test occlusion and results of surgical management of the carotid artery during tumor resection. From Parkinson RJ, Bendok BR, O’Shaughnessy BA, et al. Temporary and permanent occlusion of cervical and cerebral arteries. Neurosurg Clin N Am 2005;16:249–56. Surgery and radiotherapy are the primary treatments for head/neck cancer therapy. Yet surgical resection of areas of the larynx, pharynx, and/or tongue can severely diminish quality of life through limits on speech and swallowing.4 While radiotherapy alone may better preserve organ function, it does not always provide better rates of local control and survival.52 Chemotherapy is a promising treatment; studies of head/neck squamous cell carcinomas have shown that they may be highly responsive in a dose-related fashion to the drug cisplatin. Systemic delivery of cisplatin, however, can result in side effects like severe nephrotoxicity. Selective intraarterial chemotherapy may decrease the risk and severity of these systemic effects while permitting high-dose cisplatin delivery directly to the tumor bed by taking advantage of the angiographer’s ability to selectively catheterize distal external carotid branches. Intraarterial chemotherapy is often administered in conjunction with radiotherapy, as seen in the most well-known of the intraarterial head/neck regimens, RADPLAT, reported by Robbins et al.53–55 RADPLAT (radiotherapy and concomitant intraarterial cisplatin) also combines intraarterial chemotherapy with an intravenous systemic infusion of the competitive cisplatin antagonist, sodium thiosulfate, to further protect the rest of the body from cisplatin’s systemic toxic effects.56 In this manner, RADPLAT allows for delivery of high doses of cisplatin to head/neck tumors while limiting systemic exposure. Other applications of intraarterial chemotherapy under investigation include its preoperative use to improve the quality of head/neck tumor resection (neo-RADPLAT), its role in palliative therapy,57 and its use as a potential prognostic indicator that can guide further management of disease. Patients who completely respond to intraarterial chemoradiation may have a significantly higher overall survival rate than those who have a lesser response.57,58 Several authors have subsequently reported success with selective intraarterial chemotherapy using direct superficial temporal artery access.59–62 For benign lesions, in the rare event the tumor blood supply extends intradurally (e.g., via anterior inferior cerebellar artery [AICA] or posterior inferior cerebellar artery [PICA]), embolization is contraindicated owing to risks to the intracranial circulation.63 Specifically, ICA embolization is contraindicated when collateral blood supply is insufficient (Table 94-2), as when a patient fails a BTO or when the contralateral carotid system has previously undergone embolization or ligation. Finally, for congenital vascular malformations other than the cases indicated earlier, percutaneous sclerotherapy may be considered the primary treatment modality rather than embolization and resection. TABLE 94-2 From Osborn AG. Diagnostic cerebral angiography I. Philadelphia: Lippincott Williams & Wilkins; 1999. For malignancy, if involvement of the carotid artery is by a previously untreated case of squamous cell carcinoma, surgical resection with embolization would not be the therapy of choice. Carotid invasion is usually best treated with radiotherapy. Also, if collateral circulation is inadequate as investigated by diagnostic angiography16 and BTO with or without SPECT, preoperative embolization is contraindicated. Instead, carotid artery resection with reconstruction, though rarely performed in these cases, can be considered.43 If carotid artery reconstruction is impossible, palliative chemotherapy or hospice care may be sought. An increasing number of CBS cases are recurrent CBS. In contralateral recurrent cases, if the carotid originally affected by CBS has already been occluded or ligated, contralateral occlusion or ligation is an unavailable option.48 Endovascular occlusion is also contraindicated in any patient where the contralateral carotid artery is already occluded by atherosclerosis, and in patients who have proven CBS bleeding of the ICA/CCA systems but fail BTO. In cases where occlusion is contraindicated, a self-expandable stent should be placed.47,51 If using a stent-graft (i.e., covered stent), placement in an uninfected, unexposed area is strongly recommended. Favorable sites are often unavailable in impending or acute CBS. A near certainty of subsequent infection and a high risk of occlusion or re-hemorrhage persists when stenting in unfavorable locations51,64,65 (see Fig. 94-8).
Management of Head and Neck Tumors
Indications for Endovascular Therapy of Benign Head/Neck Lesions
Paragangliomas
Juvenile Nasal Angiofibromas
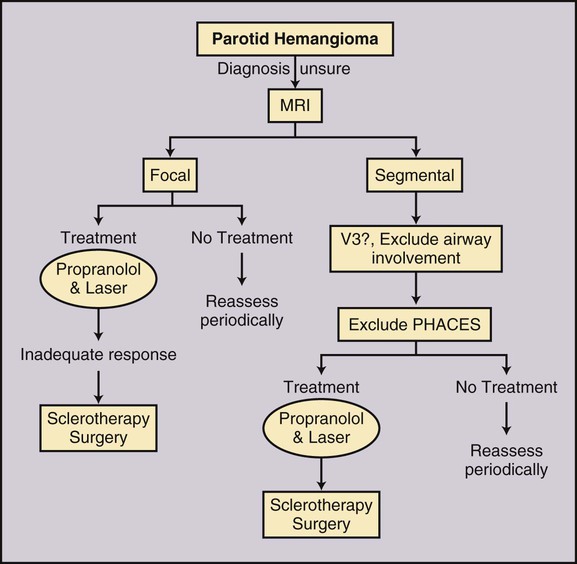
Congenital Vascular Malformations
Indications for Endovascular Therapy of Malignant Head/Neck Cancer
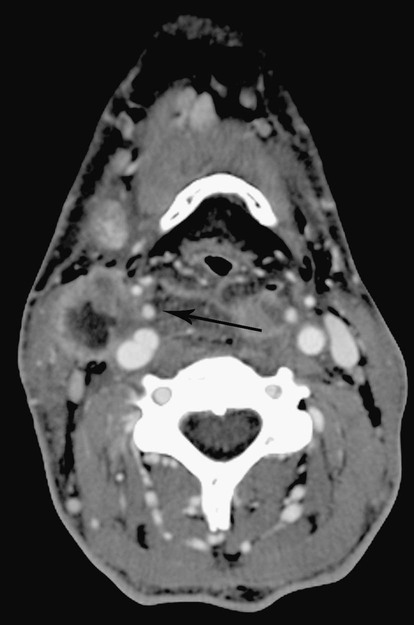
Carcinoma Involving Carotid Artery
Carotid Occlusion and Tumor Embolization Followed by Resection
Carotid Blowout Syndrome
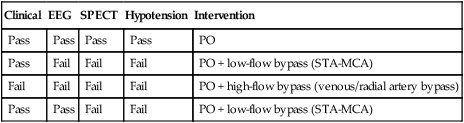
Clinical
EEG
SPECT
Hypotension
Intervention
Pass
Pass
Pass
Pass
PO
Pass
Fail
Fail
Fail
PO + low-flow bypass (STA-MCA)
Fail
Fail
Fail
Fail
PO + high-flow bypass (venous/radial artery bypass)
Pass
Pass
Fail
Fail
PO + low-flow bypass (STA-MCA)
Radplat/Intraarterial Chemotherapy
Contraindications

Carotid Blowout Syndrome
Management of Head and Neck Tumors