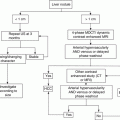
Parameter
Points assigned
1
2
3
Ascites
Absent
Slight
Moderate
Bilirubin
<2 mg/dL
2–3 mg/dL
>3 mg/dL
Albumin
>3.5 g/dL
2.8–3.5 g/dL
<2.8 g/dL
Prothrombin time (seconds over control) (INR)
<4 (<1.7)
4–6 (1.7–2.3)
>6 (>2.3)
Encephalopathy
None
Grade 1–2
Grade 3–4
There are various treatment options in the management of HCC as listed below. Decisions on treatment strategy are best taken on an individual basis through a multidisciplinary approach.
- 1.
Surgical resection
- 2.
Liver transplantation
- 3.
Radiofrequency ablation (RFA)
- 4.
Transarterial chemoembolization (TACE)
- 5.
Radioembolization
- 6.
Radiotherapy and stereotactic radiotherapy
- 7.
Systemic chemotherapy and targeted therapy
- 1.
Surgical Resection
Patients ideally suited for surgical resection are those who have disease limited to the liver, with no radiographic evidence of invasion of the liver vasculature, well-preserved liver function (Child’s A) and no portal hypertension [1–3]. Preoperative evaluation is best done with a multidisciplinary team to document adequate volume and function of the residual liver remnant. CT volumetry gives an accurate estimation of residual liver volume, and indocyanine green clearance is often used to estimate liver functional status in patients with borderline liver function. Long-term overall survival rates of ≥40% can be achieved with limited hepatic resections for small tumours (<5 cm) in patients with Child-Pugh class A cirrhosis [4].
- 2.
Liver Transplantation
For patients with localized HCC who are not candidates for resection, orthotopic liver transplantation is indicated in single lesions ≤5 cm, up to three separate lesions, not larger than 3 cm, no evidence of gross vascular invasion and no regional nodal or extrahepatic distant metastases. When these criteria are applied, a 4-year survival rate of 75% can be achieved. These criteria have become known as the Milan criteria and have been widely applied around the world in the selection of patients with HCC for liver transplantation [5].
- 3.
Radiofrequency Ablation
This technique uses high-frequency radio waves to ablate the tumour and is best suited for deep-seated small lesions (<3 cm) situated away from the hepatic hilum. The approach can be offered for tumours up to 5 cm and is associated with a local recurrence rate of 5–20%. Local ablation with RFA is recommended for patients who cannot undergo surgery or as a bridge to transplantation.
- 4.
TACE
Transarterial chemoembolization is indicated in patients with unresectable HCC that is multifocal or too large for percutaneous ablation, relatively preserved liver function (Child-Pugh A or B) and no extrahepatic disease, vascular invasion or portal vein thrombosis. TACE has been shown to provide a survival advantage over supportive care only in randomized trials [6, 7]. It is also used as a bridge to liver transplant.
- 5.
Radioembolization
Transarterial radioembolization (TARE) involves the transarterial administration of microspheres labelled with yttrium-90 (Y-90), which is a beta ray emitter having a half-life of 64.2 h and a maximum tumour penetration of 10 mm. These microspheres have a diameter of <60 μm and therefore have the ability to be shunted to the lungs or abdominal viscera. Elaborate pretreatment planning is required which includes mesenteric angiography, dosimetry planning and a transarterial macro-aggregated albumin study to look for pulmonary shunting. Transarterial radioembolization is usually preferred in the setting of portal vein thrombosis as it is associated with less embolic events [8]. Data from retrospective studies also show a trend towards better downstaging prior to transplant.
- 6.
Radiotherapy and Stereotactic Radiotherapy
Although HCC is a radiosensitive tumour, it is located in an extremely radiosensitive organ . Three-dimensional conformal radiation (3D–CRT) and stereotactic body radiotherapy techniques deliver higher doses of radiation to the tumour with less liver toxicity as compared to conventional radiotherapy. There is a lack of consensus as to appropriate indications for RT in patients with HCC. However, 3D–CRT or SBRT is a reasonable option for selected patients who are being considered for other local treatment modalities and have no extrahepatic disease, limited tumour burden and relatively preserved liver function (Child-Pugh class A or early class B).
- 7.
Systemic Chemotherapy and Targeted Therapy
Hepatocellular carcinoma is a relatively chemotherapy-refractory tumour. Although data suggests some antitumour activity of a number of chemotherapeutic agents, their use is preferable within the context of a clinical trial. Sorafenib is an oral multikinase inhibitor that has shown activity in HCC. In 2007, it was approved for treatment of unresectable HCC by the United States FDA. This was based on data from randomized trials [9, 10] which showed a modest improvement in overall survival with sorafenib. Presently, sorafenib can be recommended in unresectable HCC in Child’s A and in a select group of Child’s B patients.
3.3 Management of Cancers of the Gall Bladder and Bile Duct
3.3.1 Surgery: Gall Bladder Cancer
Surgical resection offers the only potentially curative therapy for gall bladder cancer [11]. Surgery is indicated in Stage 0–II, i.e. Tis, T1, T2, N0 where it may be curative. Periaortic, pericaval, superior mesenteric artery and/or coeliac lymph node involvement (i.e. N2 disease) has a prognosis similar to patients with distant metastasis. This group constitutes unresectable disease (Table 3.2).
Table 3.2
Gall bladder cancer—criteria for inoperability
Liver metastasis |
Peritoneal metastasis |
Involvement of N2 nodes (coeliac, peripancreatic, periduodenal, superior mesenteric nodes) |
Malignant ascites |
Extensive involvement of the hepatoduodenal ligament (infiltration of branches of the hepatic artery or portal vein) |
Presence of distant metastasis |
For T1a tumours, i.e. invading the lamina propria without muscular layer involvement, a simple cholecystectomy is adequate and offers cure rates of 73–100% [12, 13]. Patients diagnosed with incidental T1a gall bladder carcinoma after a laparoscopic cholecystectomy do not require re-resection as it does not offer any survival advantage [14].
Lymph node metastasis occurs in 15 and 62% of patients with T1b and T2 tumours, respectively [15, 16]. These patients benefit from extended or radical cholecystectomy which involves resection of the gall bladder with an en masse liver wedge resection and periportal lymphadenectomy. An intraoperative frozen section of the cystic duct margin is mandatory. Failure to achieve a negative margin at the cystic duct or frank involvement of the extrahepatic bile duct warrants an extrahepatic biliary tract excision with hepaticojejunostomy. An incidental diagnosis of T1b/T2 gall bladder cancer after simple cholecystectomy warrants a re-resection or revision radical cholecystectomy.
Stage III and Stage IVa, i.e. tumours involving adjacent organs like the stomach, duodenum colon, pancreas and the extrahepatic biliary tree, may be resectable in a selected group of patients. Surgery in this situation entails en masse resection of the involved organs and is best reserved for the fit patient at a high volume centre. Although prognosis remains guarded for this group of patients, retrospective series report favourable survival for these patients if an R0 resection can be achieved [17, 18]. However, the majority of Stage IVa tumours have involvement of the hepatic artery or portal vein rendering them unresectable. There is no role for debulking surgery; surgical exploration should only be undertaken if an R0 resection is feasible.
3.3.2 Surgery: Cholangiocarcinoma
Although surgery offers the only option for long-term control of cholangiocarcinoma, the 5-year survival rates are very poor, especially for node-positive disease even if an R0 resection is achieved. Resectability rates depend not only on tumour location but equally on the available surgical expertise as these are very specialized surgical procedures. Resectability rates for distal, intrahepatic and perihilar lesions have been reported as 91%, 60% and 56%, respectively [19].
Intrahepatic cholangiocarcinomas are managed by a formal hepatic resection and distal cholangiocarcinomas are resected with a pancreaticoduodenectomy. Perihilar tumours are the most surgically challenging. Even at high volume centres, resectability rates are less than 50%. Resection of the extrahepatic bile ducts alone leads to high rates of local recurrence either at the confluence of the hepatic ducts or at the caudate lobe branches. Addition of a hepatectomy with a caudate lobectomy improves outcomes [20, 21]. The type of surgical resection depends on the Bismuth subtype. For type I and II tumours, the procedure involves an en bloc resection of the extrahepatic ducts (with a 5–10 mm margin) with the gall bladder, regional lymphadenectomy and hepaticojejunostomy. A hepatic lobectomy is often required to achieve adequate margins on the bile ducts. Type III lesions usually require an additional lobectomy or trisectionectomy. As the caudate lobe branches are frequently involved in type II and III tumours, most centres recommend a caudate lobectomy in these patients. Extended resections involving the portal vein and/or multiple hepatic resections can be offered for the select few with type III and IV tumours at centres of excellence [22].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree