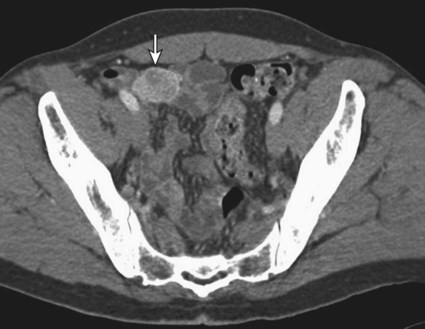
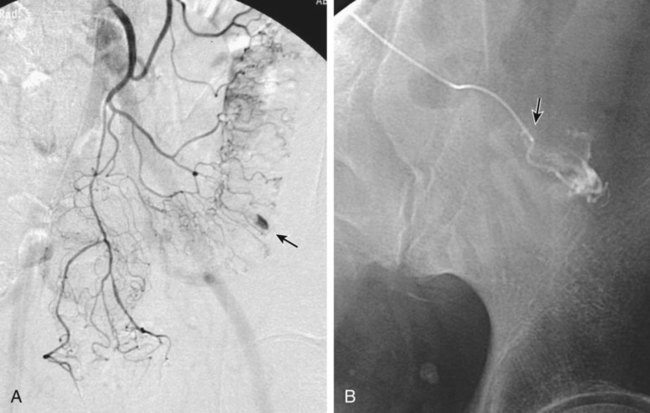
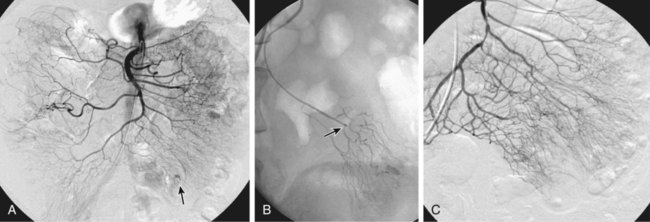
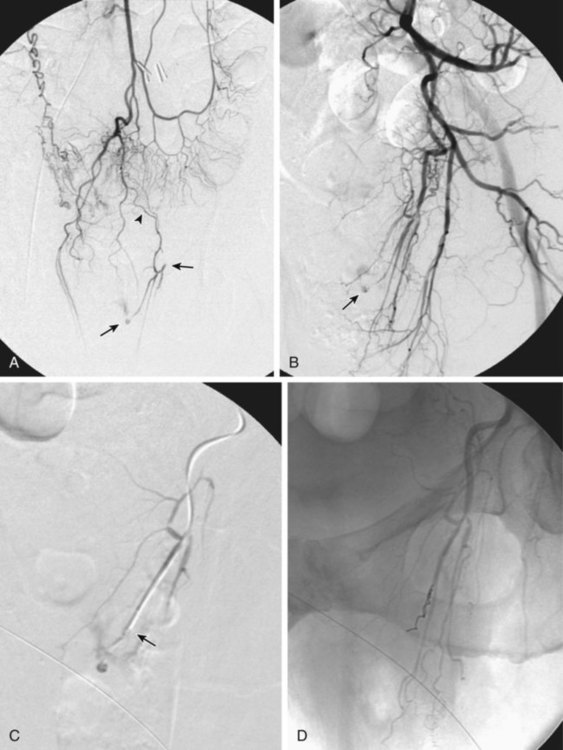
Chapter 54 Lower gastrointestinal bleeding (LGIB) is much less common than upper gastrointestinal (GI) tract bleeding; the incidence of LGIB is only around 20.4 cases per 100,000 adults per year. However, it is associated with a 10% mortality rate,1 and though less common than upper GI bleeding, it more often demands the specialized skills of the interventional radiologist. These cases are not as easily managed by endoscopic techniques, and many gastroenterologists will not attempt endoscopy while the patient is actively bleeding, owing to the difficulty in clearing blood and achieving visualization. Interventional techniques afford the opportunity to localize hemorrhage more precisely and stop bleeding less invasively than surgery. LGIB is frequently self-limiting, so the first task is to determine whether the patient is bleeding actively enough to make it likely an arteriogram will be positive. Actual output of blood is not always the best criterion. The patient can be grossly bleeding but not have hematochezia because of the large holding capacity of the colon. Alternatively, bloody output from the rectum can continue long after bleeding has stopped as old blood is expelled. Feingold et al.2 found that using pulse and blood pressure assessment alone was very predictive of a positive tagged red blood cell (TRBC) scan. In this study, 62% of unstable patients (defined as a heart rate >100 or systolic blood pressure <100 mmHg) had a positive TRBC, but only 21% of hemodynamically stable patients had positive scans. Similarly, an analysis of 88 patients showed three clinical factors that correlated with positive angiography.3 Angiograms were significantly more positive when (1) blood pressure was less than 90 mmHg (87% positive vs. 12% positive when blood pressure was higher), (2) transfusion requirements were 5 or more units of blood (84% vs. 15% for fewer transfused units), and (3) hemoglobin dropped more than 5 g/dL from prior readings (85% vs. 26% for lesser hemoglobin drops). On the other hand, angiography should not be delayed until the patient is severely hypotensive. By the time a patient goes into shock, close to 40% of blood volume has been lost.4 The threshold for deciding to do angiography should also be modified by the patient’s cardiovascular and resuscitation history. An elderly patient with moderate cardiovascular disease may need a higher perfusion pressure to avoid end-organ ischemia and thus may poorly tolerate even mild hypotension. End-organ ischemia can also be exacerbated by the decreased oxygen-carrying capacity of blood hemodiluted by resuscitation fluids. When patients are massively bleeding and hypotensive, the decision to proceed directly to angiography is fairly clear. When uncertain whether there is active bleeding, a TRBC scan may be worthwhile, since it is 5 to 10 times more sensitive than angiography at detecting bleeding. A review of over 600 TRBC scans showed that only 45% were positive.5 However, if a TRBC is negative, its higher sensitivity to bleeding provides evidence that an arteriogram will be very unlikely to demonstrate bleeding. Thus a negative scan is useful in determining the advisability of an arteriogram. Although sensitive to the presence of bleeding, TRBC scans are not as effective at localizing the bleeding site. Zuckerman et al.6 noted that TRBCs were falsely negative or provided incorrect localization in 3% to 59% of cases. In recent years, computed tomographic angiography (CTA) has been proposed as a minimally invasive way to document and localize active bleeding, with several advantages over TRBC scans. Experimental work in a swine model has shown that CT can detect bleeding at a rate as low as 0.3 mL/min.7 Early studies8–11 demonstrated the ability of CTA to document active extravasation and localize LGIB. Subsequently, CTA was shown to have 79% to 91% sensitivity, 85% to 99% specificity, and 91% to 98% accuracy for detection of GI bleeding.12–14 CTA trumps TRBC scans in that CTA can define the bleeding pathology in 52% to 75% of cases.10,15 This allows formulation of a therapeutic plan better tailored to the patient’s pathology (see Fig. e54-1 Although generally reserved for acute active bleeding, angiography can occasionally be useful in patients with chronic low-grade bleeding in whom multiple endoscopies and other tests have failed to yield the diagnosis. Rollins et al.17 reported that in patients with chronic LGIB, angiography revealed either structural lesions that could account for the bleeding or actual extravasation in 44% of their patients. Coagulopathy is a relative contraindication because it increases the risk of access site bleeding. Even with severe coagulopathy, angiography can still be done with a plan to leave a sheath in the arterial access site until the coagulopathy has been corrected. The sheath can also be used to continuously monitor arterial pressure in the postprocedure period. Alternatively, closure devices can be used to manage the arteriotomy. Another reason coagulopathy is a relative contraindication is that it significantly lowers the efficacy of embolization.18 This is because emboli usually reduce blood flow, but complete cessation of flow requires formation of some thrombus around the emboli. Angiographic catheters are intended to just engage the proximal part of the mesenteric artery, which suffices for diagnostic angiography. If bleeding is identified, these catheters are suitable for therapy only if the plan is to infuse pitressin into the main trunk of the artery. The bleeding focus is usually in the periphery of the vessel distribution, so if embolization is planned, superselective catheterization is required. A 3F microcatheter introduced coaxially through the 5F angiographic catheter is the tool of choice. Modern microcatheters are extremely flexible and often coated to decrease friction. This allows them to be advanced all the way out into the vasa recta in the bowel wall (Fig. e54-2). It is this ability to do superselective catheterization that has revolutionized interventional treatment of LGIB, since superselective catheter placement markedly improves the safety of embolization. For LGI embolization, embolic agents have evolved along with catheters. When LGI embolization was in its infancy, autologous clot and gelfoam were the available embolic agents. Their use resulted in embolization of larger branch vessels and a high risk of colonic infarction.19–21 Currently, microcoils and polyvinyl alcohol (PVA) particles are the most commonly used embolics for LGIB. Microcoils are small enough to fit through a 3F microcatheter. Their major advantage is that they are very radiopaque and can be easily visualized to allow precise placement. Their main disadvantage is that coils form immediately after exiting the catheter, and there is little option for flow direction of the coil, so the microcatheter has to be able to be maneuvered right to the desired point of embolization. Some physicians will not embolize if the catheter tip cannot be advanced out beyond the marginal artery. In Bandi’s study,22 inability to advance the microcatheter that peripherally was a cause of technical failure in 27% of their cases. PVA particles are injectable and flow directed. This allows treatment of a lesion even if the catheter cannot be advanced all the way to the bleeding site (Fig. e54-3), but also affords less control over where the emboli go. Thus a wider area of arterial occlusion is likely. Theoretically this might increase the risk of infarction, but this is not proven. To date, there have been no trials randomizing patients to either PVA or microcoils. Larger-diameter (at least 300-500 microns) PVA particles must be used. In an animal model, Kusano et al. showed that using particles smaller than 300 to 500 microns in the bowel led to a significant rate of infarction.23 Ethanol should not be used for LGI embolization. As a liquid, it penetrates to the capillary level and will lead to necrosis. In fact, there have been several case reports of colonic infarction due to ethanol that refluxed into the mesenteric circulation during renal tumor embolization.24,25 An embolic agent that shows some promise is the glue N-butyl cyanoacrylate (NBCA) (Cordis Corp., Miami, Fla.). Although this is a liquid, it can polymerize before reaching the capillary level. In one report,26 no ischemic complications followed NBCA embolization of two LGI bleeds (one middle colic, one right colic). NBCA was successful in cases where embolization with other embolic agents had failed. In elderly patients with atherosclerotic changes in the aorta, occlusion of the SMA or IMA origin is not uncommon. In cases of ostial occlusion, one should look for collateral supply. The pancreaticoduodenal arcade generally allows free communication between the SMA and celiac artery. Rarely celiac-SMA collateral flow occurs through the arc of Buhler, an embryonic communication between the main trunks. The SMA and IMA can communicate through the marginal artery of Drummond as well as the arc of Riolan (when present). The IMA can also receive collateral supply from middle hemorrhoidal braches off the internal iliacs (Fig. e54-4). Awareness of these collateral pathways is important to knowing which vessel to inject when trying to opacify a target artery with an occluded ostium. When utilizing collateral flow, delayed filming is necessary to allow the contrast to reach the target vessel. Generally, arteriography for LGIB should start with selective catheterizations (see Table 54-1). Aortography is usually not necessary. TABLE 54-1 IMA, Inferior mesenteric artery; PVA, polyvinyl alcohol; SMA, superior mesenteric artery.
Management of Lower Gastrointestinal Bleeding
Clinical Relevance
Indications
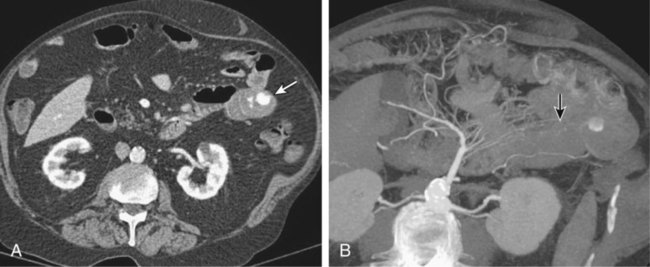
![]() ). Another advantage of CTA is its ability to depict arterial anatomy (Fig. 54-1). This should facilitate subsequent arterial catheterization as well as increase the ability to localize the bleeding site. In fact, the exquisite depiction of arterial anatomy allowed one sigmoid diverticular bleed to be embolized empirically, which previously would never have been considered in the LGI tract.16 Practical advantages of CTA over TRBC scans are that they tend to be more readily available and more rapidly performed.
). Another advantage of CTA is its ability to depict arterial anatomy (Fig. 54-1). This should facilitate subsequent arterial catheterization as well as increase the ability to localize the bleeding site. In fact, the exquisite depiction of arterial anatomy allowed one sigmoid diverticular bleed to be embolized empirically, which previously would never have been considered in the LGI tract.16 Practical advantages of CTA over TRBC scans are that they tend to be more readily available and more rapidly performed.
Contraindications
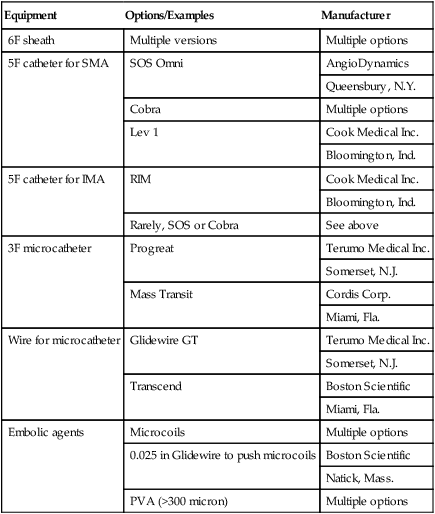
Equipment
Anatomy and Approach
Technical Aspects
Equipment
Options/Examples
Manufacturer
6F sheath
Multiple versions
Multiple options
5F catheter for SMA
SOS Omni
AngioDynamics
Queensbury, N.Y.
Cobra
Multiple options
Lev 1
Cook Medical Inc.
Bloomington, Ind.
5F catheter for IMA
RIM
Cook Medical Inc.
Bloomington, Ind.
Rarely, SOS or Cobra
See above
3F microcatheter
Progreat
Terumo Medical Inc.
Somerset, N.J.
Mass Transit
Cordis Corp.
Miami, Fla.
Wire for microcatheter
Glidewire GT
Terumo Medical Inc.
Somerset, N.J.
Transcend
Boston Scientific
Miami, Fla.
Embolic agents
Microcoils
Multiple options
0.025 in Glidewire to push microcoils
Boston Scientific
Natick, Mass.
PVA (>300 micron)
Multiple options
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine