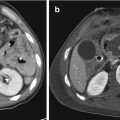
Fig. 1.1
Timely healthcare and best chance of survival. An early continuing healthcare significantly increases the probability of survival in patients with polytrauma. Mortality rates tend to decrease monotonically with life-saving primary care at the accident scene by rapid transfer to the most appropriate hospital for the definitive care. Treatment as early as 1 h or shorter after the traumatic event, especially first aid—prehospital care—platinum 10 min—can help reduce preventable deaths
1.2.2 Prehospital Care: Triage
Emergency medical services provide out-of-hospital medical care and transport patients to hospitals for extended evaluations by the diagnostic structure. Patients receiving prehospital care have a lower in-hospital mortality compared to those directly managed in the hospital and a reduced length of stay, considerably less than might be expected with; they also experience possible cost savings and reduced risks of long-term disabling sequelae. However, specific situations, particularly in the case of inefficiency of emergency department services—mainly in the phase of management and diagnostic classification at the hospital with patients receiving a standard of care that was less than good practice—increased the proportion of preventable trauma deaths.
So, when clinical teams and facilities (e.g., hospital, community, primary care) are organized to meet best practice clinical guidelines and standard services within the trauma system, each patient’s mortality could be significantly reduced [20]. The golden rule would mean that if the right team in a dedicated major trauma center with an efficient organization treats the patient, significant outcome benefits for patients with major trauma will be obtained.
Therefore, the quality of the emergency management system (EMS) as well as response times is critical to life-saving practices. To meet the growing demand of emergency medical services and prevent early deaths, it is crucial for care providers to calibrate and reduce transport time. Once an emergency call is received, the dispatch center identifies the urgency of the call, and on the basis of urgency, the center makes decisions on whether an ambulance or helicopter needs to be dispatched. This depends mainly on the distance to the site of the accident, the accessibility of the site to motor vehicles, traffic intensity, and the right hospital that is able to manage the identified injuries. European health systems provide treatment at the site of the incident, and healthcare professionals are able to correctly apply the principles of patient trauma stabilization and triage procedures and to continue care during transport aboard a land or air ambulance.
The initial assessment is indicated in the guidelines of the advanced trauma life support [ATLS] approach outlined by the American College; the ATLS is a training program for medical providers in the management of acute trauma cases. Nowadays, ATLS is widely accepted as the standard of care for initial assessment and treatment in trauma center.
It suggests to first treat the greatest threat to life. Prehospital trauma care is addressed immediately according to the ABCDE scheme, focusing on the following steps, A: Airway management; B: Breathing, ventilation, and oxygenation; C: circulation and external bleeding control; D: disability, immobilization of the spine, disability, or neurological status; and E: exposure or undressing of the patient while also protecting from hypothermia. There are conflicting views about the most suitable procedure to follow at the scene of the injury—for example, to start with a consistent, high-quality patient care at the accident site or to transfer the injured patient without delay to the trauma unit.
This dualism has had consequences in different countries.
Out-of-hospital care concepts such as “scoop and run” (rapid transport to hospital), “stay and play” (treatment and stabilization on site), or “load, go, and play” (charge quickly and stabilize the patient during the transport) have been compared in recent decades. The “stay and play” relief model, which is currently applied in European countries for closed traumas, predicts the presence of medical and paramedic figures aboard.
Staff administering Advanced Life Support (ALS) at the site of trauma results in an average trip time to the hospital of about 18.5 min. In the “scoop and run” procedure, where only Basic Life Support (BLS) is provided, emergency trips average 5 min less than the “stay and play” procedure [21].
A study undertaken to investigate changes in prehospital care for patients with severe traumatic brain injury demonstrated that the overall mortality rate did not change for the historic BLS cohort (average time on scene 7.5 min) with respect to the current ALS cohort (about four times as long as in the historic cohort) [22]. Regardless of the procedure followed in the rescue of the patient, the best common practice is to carry out life-saving operations on site as quickly as possible and to transport the patient to the most appropriate center in the shortest possible time.
In addition to the aforementioned golden hour, which indicates the importance of early relief and treatment during the first hour after the traumatic event, special attention is paid to the first “platinum 10 minutes” in which the causes of preventable deaths (e.g., airway obstruction, hemorrhagic shock) easily lead to death. The first “platinum 10 minutes” becomes important to make the golden hour effective and should be distributed as follows to make it fruitful: assessment of the victim and primary survey, 1 min; resuscitation and stabilization, 5 min; and immobilization and transport to nearby hospital, 4 min [23].
This philosophy has been likely borrowed from the military, as many battlefield fatalities occur within the first minutes post injury. Seriously injured patients should have no more than 10 min of scene-time stabilization by emergency medical personnel prior to transport to definitive care at a trauma center [24]. Two possible errors can lead to negative potential consequences at the scene of the rescue that is under- and over-triage of the patient’s injury.
Triage protocols were developed by an expert panel and indicate that over-triage is safer than under-triage because if the patient does not require care in a higher level trauma and is unnecessarily transported to such a center, this causes an overutilization of financial and human resources and can lead to overcrowding of the trauma center [25]. Over-triage rates vary in the approximate range 25–50% and may be able to be reduced while maintaining low under-triage rates [26].
Based on presenting signs and symptoms, the protocols recommend patients to one of four alternatives: (1) ambulance transport to an emergency department (ED); (2) transport to an ED by alternative means; (3) referral to a primary care provider (PCP) within 24 h; or (4) treatment at the scene only [27].
According to the “Guidelines for Field Triage of Injured Patients” published by the Centers for Disease Control and Prevention (CDC, 2011), if any of the following alterations that fall into four categories (physiologic, anatomic, mechanism-of-injury, and special considerations) are identified, it is recommended to transport the patient to a facility that provides the highest level of care within the defined trauma system [28, 29]:
Physiologic Criteria
Glasgow Coma Scale <13
SBP of <90 mmHg
Respiratory rate of <10 or >29 breaths per minute (<20 in infant aged <1 year) or need for ventilation support
Anatomic Criteria
All penetrating injuries to head, neck, torso, and extremities proximal to the elbow or knee
Chest wall instability or deformity (e.g., flail chest)
Two or more proximal long-bone fractures
Crushed, degloved, mangled, or pulseless extremity
Amputation proximal to the wrist or ankle
Pelvic fractures
Open or depressed skull fractures
Paralysis
Mechanism of Injury
Falls
Adults: >20 ft (one story = 10 ft)
Children: >10 ft or two to three times the height of the child
High-risk auto crash
Intrusion, including roof: >12 in. occupant site; >18 in. any site
Ejection (partial or complete) from automobile
Death in same passenger compartment
Vehicle telemetry data consistent with a high risk for injury
Automobile versus pedestrian/bicyclist thrown, run over, or with significant (>20 mph) impact
Motorcycle crash >20 mph
Special considerations: EMS personnel must determine whether persons who have not met physiologic, anatomic, or mechanism steps have underlying conditions or comorbid factors that place them at higher risk of injury or that aid in identifying the seriously injured patient.
Older adults
Risk for injury/death increases after age 55 years
SBP <110 might represent shock after age 65 years
Low-impact mechanisms (e.g., ground-level falls) might result in severe injury
Children
Should be triaged preferentially to pediatric capable trauma centers
Anticoagulants and bleeding disorders
Patients with head injury are at high risk for rapid deterioration
Burns
Without other trauma mechanism: triage to burn facility
With trauma mechanism: triage to trauma center
Pregnancy >20 weeks
EMS provider judgment
The ideal triage system will direct patients to the appropriate health services for their needs. Updated ambulance technology can speed up response times and improve emergency communications using high-tech wireless networks and making it possible to relay critical patient data to headquarters in real time. Nowadays, there are new apps that allow ambulance personnel to transmit key information to the trauma center, including vital signs and, more importantly, photos or video of the patient’s wounds; thus, the trauma center is able to make the necessary preparations for the patient’s arrival [30].
EMS service technologies are emerging that provide more options for healthcare providers and make patients’ lives better during ambulance transport. Boarded personnel are able to communicate via secure instant messaging with the center to obtain information regarding, for example, traffic and other obstacles; this helps to gain precious minutes when transporting patients to the trauma center.
1.3 Trauma Network
Trauma centers are specially designed to care for the most critically injured patients. New trauma centers are placed geographically with good motorway access, given that the prompt treatment of polytrauma patients by a specialized team has a higher probability of favorable outcomes. Stakeholders and healthcare planners should therefore consider this factor in the development of trauma systems [31]. In a research work comparing the availability of hospital facilities to urban and rural communities, rural communities were found to have higher risk than urban communities because they have less access to trauma centers.
The ACS-COT (Optimal Care of the Injured Patient, by the American College of Surgeons Committee) trauma center classification scheme (Level I through Level IV) is intended to assist communities in their trauma system development [32]. ACS oversees designation of trauma centers in various levels according to hospital resources and educational and research commitments. These categories may vary from state to state and are typically outlined through legislative or regulatory authorities. The different levels (i.e., Level I, II, III, IV, or V) refer to the kinds of resources available in a trauma center and the number of patients that are admitted yearly.
Level I trauma center is a comprehensive regional resource that is a tertiary care facility that is central to the trauma system. In this center, total care for every aspect of injury—from prevention through rehabilitation—is supplied, including educational and research branches.
Level II trauma centers are also able to provide complete treatment for trauma patients, but they do not have educational and research programs. Level III centers have the stabilization and initial resuscitation measures for major trauma patients. Level IV centers assure initial care and have well-functioning protocols for rapid transfer of the patients [33, 34]. Generally, the regional emergency service is organized in specialist centers of excellence (major trauma center [MTC] or “hub”) located in the regional capitals, which are equipped and staffed to provide care for patients suffering from major traumatic injuries.
An MTC must admit at least 1200 trauma patients yearly or have 240 admissions with an injury severity score of more than 15; they also must be equipped with specialist medical and nursing care. MTCs are directly connected with peripherals, radially diffused, trauma units (“spokes”) that no longer have to provide major trauma care but still play an essential role in less severely injured patients in whom transfer to an MTC may result in worse outcome.
Despite the longer transport times this entails, triage of major trauma patients to an MTC results in a 30% decrease in mortality in the first 48 h compared with transport to a non-MTC, which may be the closest medical facility [17]. This happened because the key point is not the time to reach a hospital but the efficiency of the final treatment [i.e., interventional radiology (IR) or surgery]. MTC trauma services run 24/7 for diagnostic and interventional services and provide 24/7 whole-body computed tomography (WBCT) by experienced personnel together with the image interpretation as well as 24/7 access for IR services for emergency bleeding control.
1.3.1 Inhospital Care: Primary and Secondary Survey
It is undeniable that application of time-dependent EMS interventions (e.g., airway obstruction, respiratory arrest, external hemorrhage at a compressible site) has potential positive effects on outcomes for most trauma patients. However, it is also plausible that the “golden hour” is primarily dependent on the timeliness of hospital-based interventions (i.e., initiation of definitive care after arrival at an ED) rather than out-of-hospital care [35].
The ATLS method establishes priorities in emergency trauma care by dividing the assessment of each patient’s trauma into a primary and secondary survey. The radiologist plays a key role in the early diagnosis of possible life-threatening injuries in the trauma room for defining focused treatments (primary survey) and then in the identification and definition of prognostic scores to assist in stratification of patients in clinical management (secondary survey).
1.3.1.1 The Trauma Resuscitation Team
Once the patient arrives to the hospital, the trauma team takes charge of the patient from the ambulance crew and the traumatized patient is transferred to a trauma room. The trauma resuscitation team consists of physicians, nurses, and allied health personnel, and they are all dedicated to managing the patient. Typically, trauma centers have a single level of trauma, while others may have two or three that are specifically defined in policy and monitored through the trauma quality-assurance process. The size and composition of the team may vary with hospital size, the severity of injury, and the corresponding level of trauma team activation.
A high-level response to a severely injured patient usually consists of a team with the following professionals: general surgeon, emergency physician, anesthetist, radiologist, laboratory technician, radiology technologist, and critical care nurse. The main tasks of the trauma team are the maintenance and improvement of vital functions, diagnosis and early treatment of lesions, and execution of emergency procedures. Major trauma, covering various organs and districts, is certainly the disorder/disease for which a multidisciplinary approach could provide a significant outcome. All levels are based specifically on the hospital resources available to the trauma patient as well as the patient’s physiological status. Hospital staff may rely on a report from EMS about the life-threatening injuries identified by the rescue team aboard the ambulance by application of the systematic ATLS primary survey protocol to confirm previously detected vital sign changes.
Therefore, the first step is the activation of the trauma team and to provide immediate resuscitation to the seriously injured trauma patient using hospital resources. In this way, the trauma leader continuously reevaluates the prior ATLS findings since the patient’s condition may change (e.g., deteriorate) rapidly. Usually, when a polytrauma patient is identified, the trauma team activates all resources within 15 min of notification.
Each trauma center acts according to internal protocols clearly documented by a “trauma team activation policy” with defined roles and responsibilities for each component. These protocols are subjected to continuous improvements to meet the needs of the plurality of cases encountered. Since there are a variety of hospitals at different organizational levels, no definitive list of trauma team activation criteria exists that is safely employed at all facilities. Each ED that treats polytrauma patients should develop an internal protocol for appropriate multidisciplinary team mobilization on the basis of the internal human and facility-based resources.
In Level I and II trauma centers, the highest level of activation requires the response of the full trauma team within 15 min of arrival of the patient; this includes a surgeon, emergency physician, trauma-trained nurses, imaging department team support, laboratory team support, and respiratory team support.
1.3.1.2 Primary Survey
Historically, the standard of care for trauma patients (i.e., the advanced trauma life support [ATLS] approach) outlined by the American College of Surgeons [36] indicates the guidelines for a reliable evaluation of traumatized patients. The protocol states to identify the most immediate life-threatening conditions and adopt the measures for minimizing the potential risk. The objectives of the initial evaluation of the trauma patient are as follows: (1) to rapidly identify life-threatening injuries, (2) to initiate adequate supportive therapy, and (3) to efficiently organize either definitive therapy or transfer to a facility that provides definitive therapy.
In the primary survey, the sequence and timing of the resuscitation procedures are identified by successive phases following the order A–B–C–D–E (airways–breathing–circulation–disability–exposure/environment). The initial assessment and the arrangement in the primary survey and resuscitation phases can and should be rapid (5–10 min).
A (Airway): Airways and Cervical Spine Protection
The first priority is airway patency by determining the ability of air to pass unobstructed into the lungs. An acute airway obstruction is the leading cause of death in trauma patients. Maxillofacial trauma, neck trauma, and laryngeal trauma are the most common causes of airway dysfunction. As obstruction may partially or totally prevent air from getting into the lungs, and consequent clinical signs ranging from stridor, dysphonia, wheezes, or high respiratory rates together with an altered state of consciousness (e.g., restlessness, stupor, coma) can be a consequence of a respiratory tract obstruction. The most common cause of airway obstruction in the unconscious patient is the hypotonic tongue, but foreign body upper airway obstruction, secretions in the airway, soft tissue damage, and respiratory tract irritation are all potential causes of an obstructed airway. The most basic airway maneuvers are the chin lift and jaw thrust. In a patient who has not been cleared of a cervical spine injury, these maneuvers must be done without significant neck extension. Once the basic maneuvers have been performed, the oral cavity is carefully cleaned, by aspiration of foreign bodies and liquids using electric vacuum suction, which hinders vomit and worsening of the situation. Immobilization of the cervical spine must be instituted until a complete clinical and radiological evaluation has excluded injury (Fig. 1.2).


Fig. 1.2
Immobilization of the cervical spine and maneuvers to ensure the patency of the airway
Oropharyngeal and nasopharyngeal airway devices can provide temporary return of airway patency in an unconscious patient until the airway is definitely secured though intubation. Tracheal intubation is indicated for airway protection (GCS < 9; severe maxillofacial fractures; laryngeal or tracheal injury; evolving airway loss with neck hematoma or inhalation injury) and as a conduit for ventilation (apnea, respiratory distress—tachypnea >30, hypoxia/hypercarbia) [37].
B (Breathing): Ventilation and Oxygenation
A consequential step is the immediate evaluation of the patient’s ability to ventilate and oxygenate. A thorough physical examination of the chest should be performed quickly after the initial assessment to rule out possible tension pneumothorax, massive hemorrhage, flail chest, and cardiac tamponade, which are all life-threatening conditions. According to the ATLS, the patient’s chest should be exposed to adequately assess chest wall excursion, then auscultation should be performed to assure gas flow in the lungs; then, percussion should be performed to exclude the presence of air or blood in the chest, and finally visual inspection and palpation may detect injuries to the chest wall that may compromise ventilation. A pulse oximeter can be applied to evaluate the efficiency of breathing, and if needed provide supplemental oxygen with bag-valve mask unit or tracheal intubation. In the case of flail chest/severe pulmonary contusion, pneumothorax, or hemothorax, re-expansion of alveolar volume can be obtained by performing endotracheal intubation, mechanical ventilation using a thoracentesis needle, or tube thoracostomy.
C (Circulation): Circulation and Hemorrhage Control
For the hemorrhagic shock in the injured patient who is unresponsive to the usual measures of resuscitation, pericardiocentesis treatment is applied during the primary survey. Circulation is initially assessed by simple observation of the patient, then the peculiar stress and hypovolemia response is taken into account; moreover, the traumatized patient, to compensate for a significant hemorrhage, releases a significant amount of catecholamine and increases cardiac contractility, which increases the heart rate and the systemic resistance. As blood loss progresses, mental status deteriorates, heart rate increases, blood pressure falls, and oliguria is apparent [38]. The estimated blood loss, using vital signs proposed by ATLS to manage the best resuscitation strategy, classifies the state of shock into four classes, according to the blood loss, pulse rate, and pulse pressure [39].
The patient whose persistent vital sign evaluation suggests hypotension is at significant risk for loss of 30–40% of blood volume on presentation and often leads to imminent cardiac arrest. Rapid and accurate assessment of the patient’s hemodynamic status based on clinical and hemodynamic criteria is assessed by a combination of parameters: cardiovascular (blood pressure, pulse, pulse pressure); pulmonary (oxygen saturation via pulse oximetry, respiratory rate); skin appearance (color, temperature, capillary refill); CNS (consciousness level); renal-urine output (normal 0.5 cc/kg/h in adults, 1.0 cc/kg/h in children, 2.0 cc/kg/h in neonates).
The estimated blood loss using vital signs proposed by ATLS to manage the best resuscitation strategy classifies the state of shock into four classes according to the blood loss, pulse rate, and pulse pressure [39]:
Class I: Blood Loss <15% (<750 mL); Pulse rate < 100, normal BP, normal Pulse/Pressure;
Class II: Blood Loss 15–30% (750–1500 mL); P = 100–120, normal BP, decreased PP;
Class III: Blood Loss 30–40% (1500–2000 mL); P = 120–140, decreased BP, decreased PP;
Class IV: Blood Loss >40% (>2000 mL); P > 140, decreased BP, decreased PP.
It is important to note that with the increase of blood loss, particularly when quantification of the loss amount is not feasible (e.g., trauma and occult bleeding), the vital signs that are used to guide fluid replacement in trauma patients with hypovolemic shock due to hemorrhage are not altered. In fact, in Class II, when faced with a circulating blood volume reduction of up to 30%, patients may display blood pressure values that are quite normal but with altered pulse and pulse pressure values. Patients only exhibit tachypnea, tachycardia (HR > 120), decrease in systolic BP, delayed capillary refill, decreased urine output, and a change in mental status for Class III hemorrhages, which are characterized by 30–40% blood loss (1500–2000 mL). For each class, ATLS allocates therapeutic recommendations for example, either the replacement of intravenous fluids (class I–IV) or the administration of blood products (class III–IV) [39].
It is always required to identify the presence of any source of external bleeding with a systemic approach by applying direct pressure; in the presence of uncontrolled bleeding from limbs, pneumatic tourniquets should be immediately used. All polytraumatized patients should be connected to a multi-parameter monitor in order to have a continuous reassessment of the respiratory and circulatory parameters. Two large-bore intravenous lines should be obtained to replace fluids and deliver medications. In case of hypovolemic shock, the infusion plan involves the administration of 250–500 mL warmed boluses; often, a total of 2–3 L of IV fluids is necessary, which will then need to be followed by blood transfusion bolus if hemodynamic stability is not achieved. The positive response to therapy leads to a substantial improvement of vital signs manifested through blood pressure, tachycardia, CNS-mental status normalization, urine output, and organ perfusion improvement [40].
A shock condition in traumatized patients is attributed to hemorrhage until proven otherwise; in relation to the context, of course, different and concurrent causes should be assessed: bleeding from the thorax (massive hemothorax, vascular injury, penetrating cardiac injury); abdomen (solid-organ injury [liver, spleen, or kidney], major vessel injury, or mesenteric bleeding); retroperitoneum (pelvic fracture); long bone fractures (e.g., femur); and also myocardial dysfunction after contusion due to thoracic trauma, or medullary impairment with neurogenic shock (hypotension without increase of heart rate or vasoconstriction) due to head and neck injuries.
D (Disability): Neurological Assessment
A brief neurologic exam is carried out to assess whether a serious head or spinal cord injury exists. This assesses the patient’s level of consciousness, papillary size, and reaction and possible lateralizing signs. The level of consciousness is classified according to the Glasgow coma scale (GCS) or the AVPU score. The GCS evaluates the severity of head injury by classifying three different aspects of behavioral response to external stimulation: eye opening; motoric reaction; and verbal response. The score ranges from 3 to 15, where a score of 15 represents a patient’s eyes spontaneously opening, obeying commands, and being normally oriented. The worst score is 3 points.
A decreased GCS can be caused by a focal brain injury (i.e., an epidural hematoma, a subdural hematoma, or a cerebral contusion) and by diffuse brain injuries ranging from a mild contusion to diffuse axonal injury [41]. The pupils are also examined for size, symmetry, and reactiveness to light, the spinal cord is assessed for injury by observing the spontaneous movement of the extremities and spontaneous respiratory effort. Oxygenation, ventilation, perfusion, drugs, alcohol, and hypoglycemia may all also affect the level of consciousness. Patients should be reevaluated frequently at regular intervals, as deterioration can occur rapidly, and often patients can be lucid following a significant head injury before worsening.
E (Exposure): Exposure and Thermal Protection
Trauma patients should be completely undressed for a thorough physical examination. Soon after, they should be protected from thermal dispersion. Then, the trauma patient is treated prophylactically with the administration of warmed intravenous fluids, blankets, heat lamps, and warmed air-circulating blankets as needed.
Formulation of the Patient’s Severity Index
At the end of the qualitative and quantitative assessment of all phases summarized with the acronym ABCDE, the patient’s chance of survival is calculated according to the injury severity score (ISS), which correlates the mortality, morbidity, and hospitalization time after trauma with a number varying between 0 and 75. A major trauma (or polytrauma) is described by an ISS index greater than 15 [42]. In addition to the ISS, many trauma score systems have been developed and used. For instance, the revised trauma score (RTS) [43] is the most widely used although its calculation is too complicated for easy use in the ES [44].
According to the ATLS indications, imaging is helpful during the primary survey, but the use should neither stop nor delay life-saving maneuvers. The inherent instability of the trauma patient in this setting provides a requirement for rapid imaging and accurate, timely interpretation. It is especially relevant because evaluation by history and clinical examination alone has been shown to result in misdiagnosis in 20–50% of patients with blunt polytrauma [45]. A common concept in trauma management that early intervention leads to improved outcomes is that of the “golden hour” [36]. Since its inception, the advanced trauma life support (ATLS) program has been adopted in over 60 countries and has repeatedly undergone important changes. Throughout these revisions, the role of medical imaging has evolved. The current iteration of the program includes, after the “ABCDE” of the primary survey, descriptions of a trauma series (plain film radiographs of the cervical spine, chest, and pelvis), a focused assessment with sonography for trauma (E-FAST) examination, and the selective use of MDCT. The secondary survey is essentially a head-to-toe examination with completion of the history and reassessment of progress and vital signs.
Flowchart of Diagnostic Imaging
The diagnostic procedure to be used varies according to the patient’s hemodynamic condition. An “unstable” patient is one with blood pressure < 90 mmHg and heart rate >120 bpm, with evidence of skin vasoconstriction (cool, clammy, decreased capillary refill), altered level of consciousness, and/or shortness of breath [46]. In particular, in the case of hemodynamically stable patients (blood pressure > 90 mmHg, pulse <120/min) or patients stabilized after primary resuscitation, full-body CT scan remains the gold standard in the evaluation of injured patients because it allows a detailed view of the body. In contrast, for hemodynamically unstable patients (blood pressure < 90 mmHg, pulse rate > 120/min), the time-consuming TC scan is not suggested; instead, it is suggested to use X-ray and US during the primary survey [47, 48].
X-rays and ultrasonography provide an initial diagnosis of conditions that can endanger the patient during the diagnostic phase, and in this scenario, the radiologist plays a key role at the emergency setting to provide a first effective diagnostic confirmation of potentially life-threatening clinical situations [49].
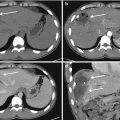
During maneuvering, resuscitators are beside the patient who is lying supine, making all the maneuvers to stabilize the patient and carrying out imaging tests such as chest X-ray (CXR) with an AP view, cervical spine X-ray with an LL view, pelvis X-ray with an AP view, and E-FAST scan (extended focused assessment with sonography for trauma). Subsequently, as mentioned above, the hemodynamically stabilized patient undergoes a TC exam that obtains a complete evaluation of all of the body parts (Fig. 1.3).


Fig. 1.3
Outline of the current algorithm for the assessment and management of polytraumatized patients
Emergency Radiology During the Primary Survey
Radiology is the key component of the trauma center, which is a determining factor for the diagnosis and subsequent treatment of trauma injuries, and therefore radiologists are a part of the trauma team. In dedicated trauma services in large hospitals, the team leader of the emergency radiology (ED) directs the evaluation and resuscitation in cooperation with general and orthopedic surgeons, physicians, radiologists, and anesthetists of the ED staff. Neurosurgeons interventions, when significant central nervous system injury is present, can be life saving. A well-integrated team should include all medical professionals involved in the patient’s care in addition to the radiologist. Often trauma patients are unconscious and uncooperative with medical staff, and this hampers the correct interpretation of the injury mechanism within the right context of the trauma event. This does not properly address the physician and radiologist toward the best-suited technique and protocol for the patient considering the technological resources available to the ED. So, radiologists undergo a significant amount of formal education to provide their expertise to the emergency staff in cooperation with other specialists to improve the quality of patient management.
Logistics of the ED put the patient at the center of the scenario; specialists in the emergency room surround the patient (Fig. 1.4). In this context, the role of the radiologist is of primary importance because he is the only specialist that has a full understanding of the final product (the images) and the knowledge of technical equipment and imaging techniques.


Fig. 1.4
The use of multidisciplinary in-hospital polytrauma teams within an Emergency and Admission Department in a context of concrete and complete collaboration improves patient outcomes
Radiology can no longer be viewed as an “add-on” to ED. Indeed, there is no case of urgency or a few cases that are not followed by an imaging act. Emergency radiology is distinguished mostly by the adaptation to any clinical patient’s situation, and the radiological response can oftentimes be the most effective, most specific, fastest, and least expensive.
Imaging services therefore must be as rigorous as the other specialties involved in the ED, and they should have the same human resources as other medical services. The powerful informatics systems introduced in the medical arena have allowed to rapidly solving complex health problems and are dependent on the development, for the main part, of the social and political interaction skills of the developer. Therefore, before being a hardware problem, the radiological emergency is a human-based problem.
The clinical radiologist orients and adapts the radiological prescription under its responsibility by an immediate interpretation, and intervening eventually on therapeutics (interventional radiologist). Efficient and optimized care is realized with the cooperation of team members that contribute to the patient’s health. Therefore, each qualified “professional” that is directly involved in the diagnostic and therapeutic management will discuss with the radiologist the choice of explorations according to the patient’s problem. Efficient patient management requires communication between team members and the radiologist. Each team member supports the patient-centered care to the best of his or her ability.
In order to minimize delay and transport, life-saving maneuvers need to be performed without stopping resuscitation—this may even require bringing mobile diagnostic apparatus to the patient’s bedside. From the emergency room, the patient is transported to the operating block in the shortest possible time; therefore, the CT room must be located within the emergency care area.
Chest X-Ray (CXR)
The plain anteroposterior chest radiograph remains the standard initial exam for the evaluation of the polytraumatized patient in the emergency room. Because of the inaccuracy of clinical signs, important thoracic problems that require possible intervention can be identified using a chest X-ray.
In cases of hemodynamic instability, the presence of respiratory failure (hypoxemia and dyspnea), or after pleural decompression or pleural drainage insertion, an ordinary chest X-ray is recommended. In all cases of blunt trauma, the patient must have a chest X-ray in the supine position in the resuscitation room since unstable spinal fractures have not been ruled out at this stage. In penetrating trauma (penetrating injuries), both from firearms and stab wounds, a chest X-ray should be taken preferably with the patient seated upright to increase the sensitivity for detecting small hemothorax, pneumothorax, or diaphragm injury.
Cervical Spine X-Ray
Cervical spine injuries are the most dreaded among all spinal injuries because of the potential serious neurological sequelae. Significant cervical spine injury is very unlikely in the case of trauma if the patient has normal mental status without neck pain, tenderness on neck palpation, neurologic signs, or symptoms referable to the neck (such as numbness or weakness in the extremities), other distracting injuries, and history of loss of consciousness [50]. However, the radiological series for excluding a cervical spine fracture requires a posteroanterior view, a lateral view, and an odontoid view. The lateral view must include seven cervical vertebrae as well as the C7-T1 interspace, allowing visualization of the alignment of C7 and T1.
According to current evidence, CT imaging of the cervical spine in polytrauma patients has replaced plain film imaging due to its greater sensitivity.
Pelvis X-Ray
Pelvic fractures resulting from motor vehicle accidents and also from falls from heights are very complex, as they imply high-energy trauma that disrupt the solid pelvic ring. These fractures are rarely isolated and are often associated with life-threatening complications such as bleeding (arterial, venous, and cancellous bone).
Up to 60% of mortality rates likely related to significant differences in fracture types have been reported [51]. Hemodynamic instability and multiple organ failure as direct consequences of pelvic hemorrhage have been identified as the primary causes of death following pelvic fracture [52]. In the prehospital exam, signs and symptoms of pelvic injury include deformity, bruising, or swelling over the bony prominences, pubis, perineum, and/or scrotum. Leg-length discrepancy or rotational deformity of a lower limb (without fracture in that extremity) may also appear. Wounds over the pelvis or bleeding from the patient’s rectum, vagina, or urethra may indicate an open pelvic fracture. Neurological abnormalities may also rarely be present in the lower limbs after a pelvic fracture [53]. Screening radiographs of the pelvis are recommended when the mechanism of injury or the degree of hemodynamic instability indicates the possibility of a pelvic fracture. According to the mechanism and severity, pelvic fractures are classified into three main patterns of injuries: anteroposterior compression, lateral compression, and vertical shear [54].
Anterior posterior compression is secondary to a direct or indirect force in an AP direction leading to diastasis of the symphysis pubis with or without obvious diastasis of the sacroiliac joint or fracture of the iliac bone. AP compression injuries cause an increased pelvic volume with any resulting hemorrhage that is unlikely to spontaneously tamponade. Pelvic wrapping therefore should be a priority in early management [55]. The AP projection, recommended by the ATLS program performed during the primary survey provides a large amount of information about the mechanism of injury. In the anterior, the AP projection can identify the presence and extent of the diastasis of symphysis pubis and/or the fracture of the obturator ring. In the posterior, the AP projection recognizes the presence and extent of dislocation of the injured side of the pelvis, dislocations of the sacroiliac joint, or fractures of L5 transverse apophysis. However, this type of projection does not help to evaluate the real dimension of the injury, especially its posterior component [56].
Lateral compression is a lateral compression force that causes rotation of the pelvis inwards, leading to fractures in the sacroiliac region and pubic rami. The lateral fractures are the most common type of pelvic fractures that are mainly associated (88% of cases) to the sacral fractures [54]. In lateral fractures, there is a reduction of the pelvic volume with hemorrhage that is more likely to spontaneously tamponade.
Vertical shear is an axial shear force that disrupts the iliac or sacroiliac junction and is combined with cephalic displacement of the fracture component from the main pelvis. In vertical shear injuries, the hemipelvis is shifted cranially, and fractures are vertically and rotationally unstable. There is a high rate of associated injuries to the torso and spine and a high rate of hemodynamic instability [55].
Other projections that can add more information are the following: the oblique outlet view, performed with patient in supine position; caudal-cranial inclination of 30° of the incidental beam centering on the pubis is useful in quantifying the cranial dislocation of the injured hemipelvis; and the oblique inlet view, which is performed with patient in supine position, caudal-cranial inclination of 30° of the incidental beam centering on the umbilicus is useful in documenting the posterior sacroiliac joint dislocation or pubic branches dislocation on AP view or the inward/outward rotation of the pelvis [56].
Focused Assessment with Sonography for Trauma (FAST) and Extended to Thorax-FAST (E-FAST)
Ultrasound (US) is an important adjunct to the primary survey of polytraumatized patients and has replaced diagnostic peritoneal washing or sometimes laparotomy in the resuscitation room because it is noninvasive, repeatable, safe, non-irradiating, inexpensive, and quick to perform [57]. There are no absolute contraindications against its use except in cases where the patient may require immediate surgery. However, before transferring the patient to the operating room for emergency laparotomy, it may be necessary to exclude pericardial tamponade or pneumothorax.
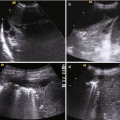
In the trauma setting, the FAST and E-FAST examinations are usually performed in hypotensive and hemodynamically unstable patients because they help to determine whether immediate surgery is needed before the patient undergoes a CT evaluation [58] (Fig. 1.5).


Fig. 1.5
The extended FAST examination (e-FAST) during the primary survey is essential for the exclusion of pneumothorax and pericardial tamponade in unstable patient
In patients with major trauma, the first-line abdominal US examination is generally performed with a FAST protocol (i.e., focused assessment with sonography for trauma), which aims to identify a free-fluid effusion within the peritoneal cavity or pericardial sac through the ultrasound exploration of four regions (subxiphoid region, right and left hypochondriac regions, pelvic cavity). The FAST scan is performed at bedside in the ER; it is usually performed with a portable machine using a lower frequency transducer, such as a 3.5–5 MHz convex array [59]. Although the effectiveness of the FAST scan to detect free intra-peritoneal fluid has been reported by many studies, the sensitivity of the FAST examination as a diagnostic test for therapeutic laparotomy accounts for only 75% (while confounded by multiple variables); similarly, its positive predictive value was only 37.3% [59]. In another review, a positive FAST exam was found to vary in the 24.2–56.3% range for penetrating trauma, while the diagnostic modality was highly specific (94.1–100.0%) but not very sensitive (28.1–100%) [60]. If the free abdominal effusion is considered as the only diagnostic finding, the rate of false negatives may further increase, since too-early scans might miss significant releases of intra-peritoneal fluid, requiring more time to accumulate [61]. Even if it is claimed that ultrasound can detect as little as 100 mL of free intra-peritoneal fluid, for a negative response it is necessary to observe the patient for at least 4–6 h and, if indicated, repeat the FAST scan or conduct a CT scan [62]. Some clinicians incorporate inferior vena cava (IVC) evaluation into the FAST examination to help determine the patient’s volume status and fluid responsiveness [63].
Ultrasound may also be technically limited in the traumatized patient due to bowel gas, obesity, subcutaneous emphysema, or patient positioning. Other limitations to FAST assessments include its poor sensitivity in detecting the hemoretroperitoneum, parenchymal traumatic injuries, and almost bowel and mesenteric injuries detection; the latter two are both diagnosable with a CT scan.
Although FAST has a high specificity, false-positive results can be encountered in patients with a history of ascites or inflammatory processes in the abdomen or pelvis or even ovarian cyst rupture.
The Consensus Conference recommends that hemodynamically unstable patients with positive FAST examination should generally be followed by a laparotomy, while a search for extra-abdominal sources of hemorrhage should follow a negative exam [64].
In hemodynamically stable patients, a positive FAST result should be followed by a TC exam to better define the nature of the injuries. For negative FAST scan cases, a period of monitoring for at least 6 h, serial FAST scans, or further investigations (e.g., CT scan or peritoneal lavage) are recommended.
In many trauma centers, the use of the FAST technique is extended to the thorax (Extended-FAST) for the detection of hemothorax and pneumothorax, including the presence of hemopericardium (whose evaluation is already performed with a FAST scan) through the execution of some standard scanning with a convex and linear probe (transducer) [58]. Thoracic ultrasound (e-FAST) is defined as a rapid and accurate first-line bedside diagnostic modality for the diagnosis of pneumothorax in unstable patients with major chest trauma during the primary survey in the emergency room [58].
The diagnostic performance of ultrasound is high (i.e., 77% sensitivity, 99.8% specificity, 98.5% positive predictive value, 97% negative predictive value, and 97.2% accuracy). This sensitivity value is statistically significantly higher than that of supine chest radiograph (approximately 50%, as reported in the literature). According to anti-gravity laws, PNX air in the pleural space tends to accumulate in the least-dependent part of the chest [65, 66]. When the patient lies in the supine position, the area of interest corresponds to the anterior and inferior part of the chest on both sides of the thorax, which is approximately the third–fourth intercostal space between the parasternal and the mid-clavicle lines [67, 68]. The probe should be placed in the intercostal acoustic window of the located area.
The parietal pleura appears as a thin echogenic horizontal line located between and below two adjacent ribs. Sometimes, it is necessary to scan more intercostal spaces by moving the probe laterally and inferiorly in order to evaluate the extension of PNX or to confirm the diagnosis. Sonographic signs that can help in the diagnosis of a pneumothorax are the absence of lung sliding (the visualization of lung sliding has a 100% negative predictive value), lung pulse, loss of B lines, and identification of the lung point (100% specificity) [69].
Patients in physiologic extremis and suspected of having PTXs on physical examination should undergo immediate tube or needle thoracostomy without awaiting imaging studies [70] (Fig. 1.6). The limitations in the evaluation for pneumothorax include main-stem bronchus intubation, severe chronic obstructive pulmonary disease, or other lung pathology that inhibit adequate visualization of lung sliding, and small apical or localized pneumothoraces may not be visualized [63].
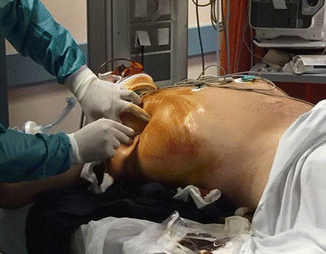

Fig. 1.6
The use of the e-Fast protocol allows to place as promptly as possible a pleural drainage in a patient with pneumothorax and subcutaneous emphysema
Contrast-Enhanced Ultrasound (CEUS)
Ultrasonography has in recent years found new application areas through the use of contrast enhancement based on microbubbles [48]. This method has many advantages mainly due to the fact that avoiding ionizing radiation represents a safe tool—particularly in selected series, including pediatric patients and females of reproductive age [71].
Its application for children is still considered off-label, and it is allowed only after the parents (or legal guardians) have been adequately informed and given their specific consent. CEUS also exhibits an advantage compared to contrast-enhanced MRI and CT because it can be used in patients with renal failure or allergy [72]. The CEUS technique can be carried out in a variety of scenarios, including bedside, operating room, and trauma suite [71]. Also, compared to other techniques, it is not expensive. There is still debate for how to integrate CEUS in the trauma patient workup [73, 74].
Although it is considered to be more sensitive and accurate than baseline US and almost as sensitive as CT in the identification and characterization of solid-organ lesions in blunt abdominal trauma, the CEUS use in the primary survey is limited by several factors such as the lack of panoramic view, typical of CT, and, like conventional US imaging, insensitivity in depicting diaphragmatic rupture, bowel, or mesenteric traumatic injuries [75]. Moreover, CEUS is strongly operator dependent, requiring dedicated contrast agent-specific software, and it takes a long time to be performed [76]. An excellent correlation was found between the size of the traumatic injury (as shown at CT) and the related CEUS findings with respect to hepatic traumatic injuries, advocating its employment as a first-line examination in mild blunt abdominal trauma and in pediatric patients and leaving CT as the gold standard for polytrauma and major trauma [74].
Nowadays, contrast-enhanced ultrasound (CEUS) has an important role in the follow-up of conservatively treated traumatic injuries of the abdominal parenchymal organs (liver, spleen, and kidneys) or as a first-line examination in low-energy or mild isolated abdominal trauma [76, 77]. It can integrate conventional US imaging in the triage of hemodynamically stable patients, in particular with low-energy abdominal trauma; also, it can be routinely used as a completion of a US FAST examination to scan severe trauma patients in the assessment of solid-organ injuries [78]. This happens especially when the use of CT exposes patients to excessive radiation dose. In some departments, protocols for the follow-up of blunt abdominal trauma that are conservatively treated include a CEUS at 24 and 72 h after trauma as well as CEUS and MRI after 1 month [79].
CEUS cannot replace abdominal CT, but it represents a noninvasive and repeatable imaging tool that is capable of providing a reliable assessment of trauma severity and expediting the patient’s treatment [71].
1.3.1.3 Secondary Survey
Once any life-threatening problems have been identified using diagnostic procedures during the primary survey, hemodynamic stability is re-established through a series of therapeutic maneuvers (plasma expanders, fluid recovery, drainages). The effectiveness of vital organs are then maintained, and the secondary survey identifies other injuries with the definitive treatment. To this end, in accordance with the ATLS protocol, a comprehensive head-to-toe physical assessment is carried out to detect other significant but not immediately life-threatening injuries that were not detected or managed during the initial assessment and resuscitation.
A brief medical history of the patient is obtained according to the procedure summarized in the acronym SAMPLE (S—Signs/symptoms, A—Allergies, M—Medications, P—Pertinent past medical history, L—Last oral intake, E—Events leading to disease or injury) and a reassessment of all vital signs (pulse, respirations, skin signs, pupils, and blood pressure). Heart tones and peripheral pulses are also checked. If any clinical deterioration is detected during monitoring of the vital signs, the primary survey must be repeated immediately and measures must be taken to rectify the problem.
The head-to-toe examination takes no more than 2–3 min and identifies and urgently treats lacerations, bruising, swellings, bleedings, discolorations, bone protrusions, tamponading bleeding, and splinting fractures.
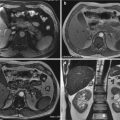
At this stage, the role of trauma radiology, essentially based on the use of CT, is important to depict life-threatening diagnoses, properly determine the stage, help the medical team to suggest treatment options, debate prognosis, and take the prognostic scores that can dictate a surgical or conservative treatment. Finally, CT scanning remains the gold standard in terms of radiological assessment for diagnosing abdominal injuries during the secondary survey for both hemodynamically stable patients and patients stabilized after primary resuscitation.
Radiology in the Secondary Survey
Whole-Body Computed Tomography (WBCT) in Trauma
Current scanners are capable of rendering submillimeter resolution images of the entire body in a matter of seconds [80]. Among the long list of advantages derived from MSCT technology for obtaining useful imaging information, we enucleate three: (a) shorter scan time, (b) extended scan range, and (c) improved longitudinal resolution, particularly when 3D post-processing is part of the clinical protocol. The ability to acquire volume data also paved the way for the development of three-dimensional (3D) image processing techniques, such as multiplanar reformation (MPR), maximum intensity projection, surface-shaded display, and volume-rendering techniques, which have become a vital component of medical imaging today. CT has become an especially vital component of patient evaluation in the ED and is helped by the increased diffuse use of picture archiving and communication systems (PACSs), teleradiology, and voice recognition software, which facilitate all the rapid image dissemination and interpretation [80–83]. CT is well suited for the ED: it provides rapid, minimally invasive, high-resolution imaging that can quickly direct patients toward further treatment. Factors that might limit the growth of CT use include cost-containment efforts [84], intervention to improve evidence-based guideline adherence [85], and concerns regarding radiation exposure [86].
WBCT is a sensitive and comprehensive tool for the diagnosis of a wide range of traumatic injuries, especially in the severely injured patient [87–91].
Contrary to selectively performing CT scans on one or more body regions, a WBCT scan comprises a CT of the head without intravenous contrast, cervical spine (acquired before, or following, intravenous contrast), and chest, abdomen, and pelvis following intravenous contrast. In the largest systematic review and meta-analysis that determined the odds of mortality in trauma patients, patients who underwent a WBCT scan were less likely to have a fatal outcome compared to those who received selective CT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree