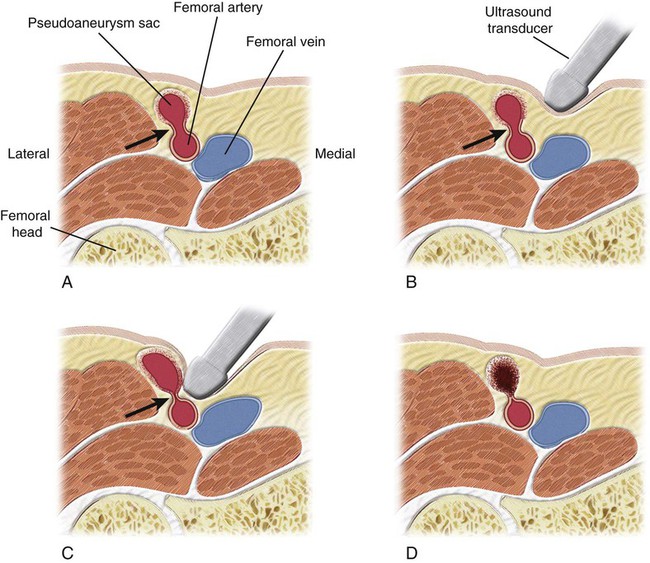
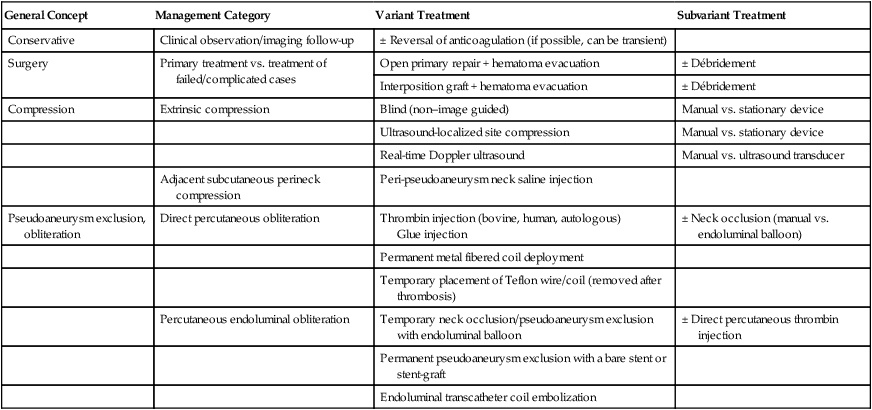
Arterial pseudoaneurysms are the most common complication (61%) of interventions requiring femoral artery catheterization (Table 40-1).1–3 The overall incidence of postcatheterization pseudoaneurysms ranges from 0.11% to 1.52%.4–10 In six studies involving 107,052 femoral catheterizations, 757 patients (0.71%) exhibited postcatheterization pseudoaneurysms.4–10 The incidence of access pseudoaneurysms increases with transcatheter therapeutic interventions (3.5%-5.5%) as compared with studies confined to diagnostic arterial catheterization (0.1%-1.1%).1,4,11 Because of the ongoing paradigm shift in transcatheter endoluminal interventions as opposed to traditional open surgical interventions, the incidence of postcatheterization pseudoaneurysms is on the rise, with approximately 15,000 femoral pseudoaneurysms diagnosed in the United States annually as of the year 2000.1,4,5,9,11–17 TABLE 40-1 Type of Arterial Catheterization Complications Requiring Intervention Data from Lumsden AB, Peden EK, Bush RL, Lin PH. Complications of endovascular procedures at the target site. In: Ouriel K, Katzen BT, Rosenfield K, editors. Complications in endovascular therapy. New York: Taylor & Francis; 2006. p. 29–53. Postcatheterization pseudoaneurysms are associated with increased morbidity (Table 40-2).1,4,11 Numerous methods, including variants within each method, have been described for the management of postcatheterization pseudoaneurysms (Table 40-3; also see Table e40-1). For the purposes of this chapter, the two most commonly described approaches—pseudoaneurysm compression and direct percutaneous thrombin injection and their variant techniques—will be discussed. The limited role of stent-grafts will also be mentioned. TABLE 40-2 Type of Complications Caused by Postcatheterization Pseudoaneurysms DVT, Deep venous thrombosis; PsA, pseudoaneurysm. TABLE 40-3 General Approaches to Management of Postcatheterization Pseudoaneurysms and Their Variant Techniques AVF, Arteriovenous fistula; PFA, profunda femoris artery; PsA, pseudoaneurysm. Pseudoaneurysms that are most amenable to compression are small pseudoaneurysms less than 2 weeks old with long accessible necks, in a patient not taking anticoagulants who has intact overlying skin. Some authors report that large pseudoaneurysms are associated with a lower success rate or require longer compression times.18–20 In addition, patients receiving anticoagulation therapy represent 64% to 100% of reported failures in studies of patients undergoing compression therapy.10,18–24 In eight reports describing a total of 684 cases with detailed analysis of failures (133 failures), 100 failures (75%) were associated with anticoagulation therapy.10,18–24 Poor patient/lesion candidates for compression therapy include (1) pain intolerance/painful pseudoaneurysms (25%-34% of failures), (2) morbid obesity (13% of failures), (3) large pseudoaneurysms obliterating adjacent vascular structures (19% of failures), (4) associated arteriovenous fistulous component (1%-2% of pseudoaneurysms), (5) superadded infection or overlying skin breakdown, and (6) unstable patients (2% of failures).18,22,25,26 Poor candidates for compression represent approximately 10% of all patients and 50% of intent-to-treat technical failures.7,18,27–31 Contraindications to direct percutaneous thrombin injection therapy include (1) infected pseudoaneurysms or overlying skin erosion/breakdown, (2) ruptured pseudoaneurysms, (3) associated arteriovenous fistulous component, (4) associated ipsilateral deep venous thrombosis (DVT), and (5) previous treatment/exposure to bovine thrombin because of concern for allergic reactions (relative contraindication).32 Little equipment is required other than the ultrasound transducer for real-time ultrasound compression. Institutions that adopt ultrasound-guided compression may use stationary (vice-like) mechanical compression devices such as the FemoStop (Femoral Compression System, RADI Medical Systems AB, Uppsala, Sweden, distributed by Radi Medical Systems, Inc., Reading, Mass.).29,33–35 TABLE e40-1 General Approaches to Management of Postcatheterization Pseudoaneurysms and Their Variant Techniques Varying preparations of thrombin, fibrinogen, and fibrin have been used.36–43 The following are some of the more commonly used products. • Gentrac Inc., for Johnson & Johnson Medical Inc., Middleton, Wis. • Jones Medical Industries, St. Louis, Mo. • Vascular Solutions, Bochum, Germany. Sterilized and virus-inactivated human thrombin, tissue sealant to be defrosted (500 U/mL): Autologous centrifuged and suspended human thrombin: Most authors use a linear array transducer ranging in frequency between 5 and 7.5 MHz, and use a lower-frequency transducer (3.5-4.0 MHz) for morbidly obese patients or those who have excessively large overlying hematomas.32,36 Some authors use higher-frequency transducers (7.5 to 12 MHz).32,44 Numerous variations of the compression management of access pseudoaneurysms have been described. One variable is the guidance method, which includes blind compression (by palpation and without image guidance),6,35,55 ultrasound examination to determine the ideal site for subsequent compression,6,29,33,35,56,58 and real-time ultrasound-guided compression.17,18,20,26,56,57,59 A second variable is the means with which the pseudoaneurysm neck is compressed, including manual/digital compression,43,56,58,60 use of stationary (vice-like) contraptions such as a FemoStop device,29,33,35 and finally, use of the ultrasound transducer itself to compress the pseudoaneurysm neck.18,20,51,52,59,61,63 Before pseudoaneurysm neck compression is applied, baseline distal arterial pulses are determined. Ankle-brachial indices (ABIs) may even be calculated. Via color-flow Doppler sonography, the pseudoaneurysm is carefully evaluated for overall size, pseudoaneurysm neck location, dimensions, and hemodynamics. The relationship of the pseudoaneurysm with the supplying femoral artery is also evaluated, in addition to associated arterial injuries such as arteriovenous fistulous components. The overlying skin is likewise evaluated for necrosis and infection. Assessment is also made of the direction required to apply pressure to occlude flow into the pseudoaneurysm sac. The ideal trajectory for applying pressure is one that avoids gross compression of the pseudoaneurysm sac itself, as well as the adjacent normal vasculature (femoral vein and artery) (Fig. 40-1). Some authors routinely use moderate sedation in patients undergoing pseudoaneurysm neck compression. For those who do not routinely sedate patients, 25% to 34% of their patients eventually require moderate sedation to tolerate the pseudoaneurysm compression.18,27,64 The ultrasound probe is positioned directly over the pseudoaneurysm neck, and downward pressure is applied until flow into the pseudoaneurysm ceases (see Fig. 40-1, B and C). The ideal degree of pressure will abolish flow into the pseudoaneurysm and, if avoidable, not compress the adjacent femoral artery. In some patients, temporary occlusion of the femoral artery is unavoidable, but there are usually no untoward sequelae.65 Compression of the femoral vein is unavoidable when the pseudoaneurysm swings medially and overlies the femoral vein. If the pseudoaneurysm neck is not clearly identified by color-flow Doppler examination, ultrasound-guided compression directed at the pseudoaneurysm sac itself can be performed until cessation of flow in the pseudoaneurysm is achieved.65 The pseudoaneurysm neck is compressed intermittently for intervals extending between 6 and 20 minutes.7,23,25,34,66–68 It is not uncommon during ultrasound-guided compression to lose the ideal position over the pseudoaneurysm neck, thus necessitating adjustment of the ultrasound transducer’s position. Between pressure applications, the pseudoaneurysm is evaluated for flow by color-flow Doppler ultrasound. If the pseudoaneurysm has thrombosed, the procedure is deemed technically successful (see Fig. 40-1, D). If there is still residual flow, a repeat compression interval is performed. Most operators will not exceed three or four such intervals. In addition, it is not uncommon for patients to not tolerate repeat compression intervals/sessions. The technique of direct percutaneous intrapseudoaneurysm injection of thrombin or tissue fibrin sealant varied considerably in earlier reports. The two main variants were the site of needle application (see Fig. e40-1 Insertion of the needle tip into the pseudoaneurysm neck for injection of thrombin has been performed in an attempt to rid the thrombin clot formed around the needle tip by flow carrying it away from the needle tip and neck into the pseudoaneurysm sac (see Fig. e40-1, A)
Management of Postcatheterization Pseudoaneurysms
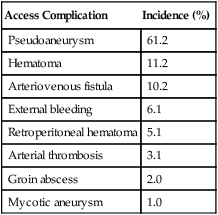
Access Complication
Incidence (%)
Pseudoaneurysm
61.2
Hematoma
11.2
Arteriovenous fistula
10.2
External bleeding
6.1
Retroperitoneal hematoma
5.1
Arterial thrombosis
3.1
Groin abscess
2.0
Mycotic aneurysm
1.0
Complication
Descriptive Incidence
Large, painful thigh/groin hematoma limiting patient ambulation
Common
Overlying skin erosion/breakdown
Not common (large PsAs)
Associated/superadded infection
Not uncommon (most subclinical)
Venous stasis/leg edema/DVT
Not common
Sensory-motor femoral neuralgia
Not uncommon with large PsAs
Anterior abdominal wall hematoma
Uncommon
PsA rupture and bleeding
Rare
Management Approach
Indications
Problems/Disadvantages
Observation
Asymptomatic patients
Requires frequent Doppler examination
Small PsA (<1.8-2 cm in diameter, <6 cm in volume)
Reduced patient activity (increased morbidity)
No anticoagulation therapy
Not inexpensive in view of above 2 points
Limited indications
Surgery
Historically: standard management
High complication rate (11%-32%)
Currently: when minimally invasive methods fail
Associated overt infection
Complex (associated) vascular injuries (AVF component)
Most are wound infection/dehiscence, bleeding, and femoral neuralgia
Endoluminal coiling
PFA branches
Circumflex branches
More involved and probably more expensive than other minimally invasive approaches
Aesthetic problems (nest of coils subcutaneously)
Temporary endoluminal balloon exclusion of PsA
Wide and short PsA necks; 15% of PsAs thrombose within minutes
More involved and probably more expensive than other minimally invasive approaches
Requires heparinized saline infusion through balloon shaft to maintain distal patency
Endoluminal stent-graft exclusion of PsA
PsA originating above inguinal ligament
No infection
More involved and more expensive than other minimally invasive approaches
Limited application/indications
Direct percutaneous coil or glue into PsA
Potentially all patients
Preferably (if ever resorted to) small PsAs (aesthetics)
More expensive than thrombin injection
Aesthetic problems (nest of coils/lump of glue subcutaneously)
Indications
Pseudoaneurysm Compression
Contraindications
Pseudoaneurysm Compression
Direct Percutaneous Thrombin Injection
Equipment
Pseudoaneurysm Compression
General Concept
Management Category
Variant Treatment
Subvariant Treatment
Conservative
Clinical observation/imaging follow-up
± Reversal of anticoagulation (if possible, can be transient)
Surgery
Primary treatment vs. treatment of failed/complicated cases
Open primary repair + hematoma evacuation
± Débridement
Interposition graft + hematoma evacuation
± Débridement
Compression
Extrinsic compression
Blind (non–image guided)
Manual vs. stationary device
Ultrasound-localized site compression
Manual vs. stationary device
Real-time Doppler ultrasound
Manual vs. ultrasound transducer
Adjacent subcutaneous perineck compression
Peri-pseudoaneurysm neck saline injection
Pseudoaneurysm exclusion, obliteration
Direct percutaneous obliteration
Thrombin injection (bovine, human, autologous)
Glue injection
± Neck occlusion (manual vs. endoluminal balloon)
Permanent metal fibered coil deployment
Temporary placement of Teflon wire/coil (removed after thrombosis)
Percutaneous endoluminal obliteration
Temporary neck occlusion/pseudoaneurysm exclusion with endoluminal balloon
± Direct percutaneous thrombin injection
Permanent pseudoaneurysm exclusion with a bare stent or stent-graft
Endoluminal transcatheter coil embolization
Direct Percutaneous Thrombin Injection
Thrombin and Tissue Adhesives
Ultrasound Transducer
Technique
Pseudoaneurysm Compression
Largely Adopted Real-Time Ultrasound-Guided Pseudoaneurysm Neck Compression Technique
Direct Percutaneous Thrombin Injection
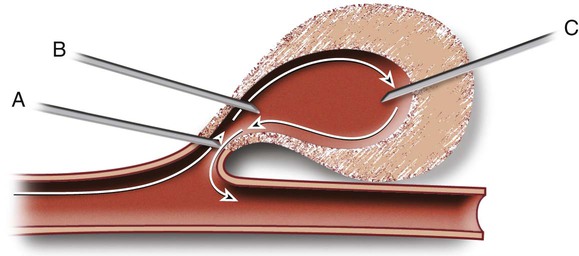
![]() ) and whether to occlude the neck. Over the past decade, the technique has been refined, and numerous authors have recently described a more uniform and simplified method to obliterate postcatheterization pseudoaneurysms with direct thrombin injection5,32,36,41,45,47,49,73–77 (see later discussion).
) and whether to occlude the neck. Over the past decade, the technique has been refined, and numerous authors have recently described a more uniform and simplified method to obliterate postcatheterization pseudoaneurysms with direct thrombin injection5,32,36,41,45,47,49,73–77 (see later discussion).
![]() . However, this location has largely been abandoned because of concern for an increased risk of femoral artery thrombin embolization. A second needle tip insertion site is at the juncture of the pseudoaneurysm neck and the pseudoaneurysm sac (see Fig. e40-1, B).
. However, this location has largely been abandoned because of concern for an increased risk of femoral artery thrombin embolization. A second needle tip insertion site is at the juncture of the pseudoaneurysm neck and the pseudoaneurysm sac (see Fig. e40-1, B).![]() The hypothetical advantage of this site is that blood flow at this juncture carries the thrombin clot that forms around the needle tip into the pseudoaneurysm neck, without the risk of femoral artery embolization.74 However, this precise site for needle tip placement can be clearly identified in only 42% of patients with the use of ultrasound contrast agents.74 The added expense of ultrasound contrast agents, the increased time needed to perform an ultrasound contrast–enhanced evaluation, and the increased technical challenge to place the needle tip at that precise location make this needle tip placement site unpopular. Furthermore, the safety (low risk of femoral embolization; Tables 40-4 and 40-5) achieved by placing the needle tip at the fundus (authors’ definition
The hypothetical advantage of this site is that blood flow at this juncture carries the thrombin clot that forms around the needle tip into the pseudoaneurysm neck, without the risk of femoral artery embolization.74 However, this precise site for needle tip placement can be clearly identified in only 42% of patients with the use of ultrasound contrast agents.74 The added expense of ultrasound contrast agents, the increased time needed to perform an ultrasound contrast–enhanced evaluation, and the increased technical challenge to place the needle tip at that precise location make this needle tip placement site unpopular. Furthermore, the safety (low risk of femoral embolization; Tables 40-4 and 40-5) achieved by placing the needle tip at the fundus (authors’ definition![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Management of Postcatheterization Pseudoaneurysms