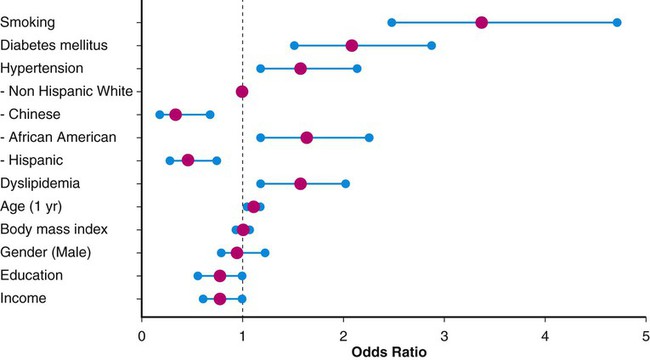
This chapter will focus on the management of peripheral artery disease (PAD), defined as atherosclerosis of the iliac and lower extremity arteries. PAD confers risk for overall mortality and cardiovascular mortality, especially among those with accelerated functional decline. A sensitive and inexpensive method to diagnose PAD is use of the ankle-brachial index (ABI), calculated by dividing the highest ankle pressure of each leg by the highest brachial pressure.1 In a prospective evaluation of a total of 6647 patients, after adjusting for age, sex, ethnicity, education, income, traditional cardiovascular disease (CVD), and biomarkers, both a low ABI (<1.00; hazard ratio [HR] 1.77; 95% confidence interval [CI] 1.31-2.40) and a high ABI (>1.40; HR, 1.8; 95% CI, 1.00-3.43) were associated with incident CVD events. McDermott et al. measured the impact of functional decline among 440 patients with PAD who completed a 6-minute-walk test at baseline and annually for 2 years. A total of 102 participants (23.2%) died during a median follow-up of 44.5 months, and those in the lowest tertile of functional decline had the worse all-cause mortality (HR, 2.16; 95% CI, 1.28-3.64) and cardiovascular disease mortality (HR, 2.45; 95% CI, 1.08-5.54).2 Although the majority of patients with PAD do not present with typical claudication, being asymptomatic is not an indicator of improved prognosis.3 Persons with PAD who have never experienced exertional leg symptoms have poorer functional performance, poorer quality of life, smaller calf muscle area, and higher calf muscle fat compared with sedentary persons with intermittent claudication.4 This stresses the need for screening selected groups of patients. The PAD 2010 performance measures recommend measuring ABI in patients older than age 18 who have walking impairment or claudication, patients with lower extremity nonhealing wounds, patients aged 50 to 69 who have a history of smoking or diabetes, and all patients older than age 70.5 Predominantly the same risk factors that contribute to coronary artery disease (CAD) and cerebrovascular disease (e.g., hypertension, chronic kidney disease, hyperlipidemia, diabetes mellitus, current or prior history of smoking) also lead to development of PAD.6,7 Patients with chronic kidney disease (CKD) are more likely than patients without CKD to have PAD and PAD complications, including critical limb ischemia and amputations.6,8,9 Given these overlapping risk factors, the majority of patients with PAD also have CAD. The risk factors are often determinants of overall cardiovascular health, including peripheral vascular disease. Non-modifiable risk factors include advancing age, gender, and race. African Americans have a threefold higher likelihood of PAD in age- and gender-matched models (odds ratio [OR] 3.1; 95% CI, 1.5-6.4).10 Compared with men, women have accelerated functional decline and greater mobility loss; these sex differences may be attributable to smaller baseline calf muscle area among women with PAD.11 Figure e18-1 depicts the corresponding ORs for traditional PAD risk factors based on data from the Multi-Ethnic Study of Atherosclerosis (MESA), which included 6814 men and women from multiple U.S. communities recruited from July 2000 to August 2002.12 Multiple biomarkers are emerging as potential indicators of disease progression. Several studies show that elevated biomarker levels are associated with increased cardiovascular event rates and mortality in persons with PAD.13 Furthermore, higher levels of interleukin (IL)-6, D-dimer, soluble vascular cell adhesion molecule (sVCAM)-1, C-reactive protein (CRP), and homocysteine are associated with slower walking velocity and shorter distances achieved in the 6-minute-walk test (a test of walking capacity) even after adjustment for ABI, comorbidities, and other potential confounders.14,15 However, not all of these serum biomarker tests are widely available, and studies aimed at reducing levels of such biomarkers have not resulted in improved outcomes. Indeed, their greatest utility may be as surrogates for disease severity. Thus, the approach to therapy is restricted to modifiable PAD risk factors: general lifestyle modification, tobacco cessation, hypertension management, cholesterol reduction, and diabetes management. These will be the focus of the remaining discussion. Lifestyle modification, including regular exercise, maintaining ideal body weight, and moderate consumption of alcohol, have been evaluated as first-line medical therapy for vascular disease. In general, patients with PAD and claudication have poor nutrition, with diets particularly high in saturated fat, sodium, and cholesterol and low in fiber, vitamin E, and folate.16 High dietary fiber intake may result in overall lower body mass index (BMI) and elevated glucose levels, blood pressure, and homocysteine concentrations.17 However, a reduction in total fat intake has not been independently proven to lower cardiovascular risk.18 In addition, intake of dietary antioxidants including vitamins C, E, and β-carotene have not proved beneficial in preventing cardiovascular disease.19 Regular exercise has been found to decrease cardiovascular mortality and improve functional capacity in affected patients.20 Both the performance measures and guidelines for managing PAD patients sponsored by the American College of Cardiology (ACC) and the American Heart Association (AHA) recommend a supervised exercise program as the initial form of treatment in those with claudication.3,5,21 Because of improved collateral flow and likely enhanced endothelial function, muscle metabolism, mitochondrial function, and decreased inflammation, exercise therapy improves walking capacity in this patient group.21,22 The optimal exercise program for improving claudication pain distances in patients with PAD uses intermittent walking to near-maximal pain during a program of at least 6 months.23 Exercise therapy offers greater improvement in the symptoms of PAD than available pharmacotherapy.21 In a meta-analysis including 21 trials evaluating exercise therapy for claudicant patients, the distance (mean ± SD) to onset of claudication pain increased 179% from 125.9 ± 57.3 m to 351.2 ± 188.7 m (P < .001), and the distance to maximal claudication pain increased 122% from 325.8 ± 148.1 m to 723.3 ± 591.5 m (P < .001).23 Until recently, it had been difficult to measure the value of supervised exercise compared to revascularization. A total of 111 patients with aortoiliac peripheral artery disease were randomized to optimal medical care alone or in addition to stenting or supervised exercise. At 6 months the primary end point of peak walking time measured in minutes was best among the patients in the supervised exercise group (mean change versus baseline: 5.8 ± 4.6, 3.7 ± 4.9).24 The added value of stent revascularization among patients on supervised exercise and optimal medical therapy is yet to be determined. Every year, more than 400,000 people in the United States die prematurely from smoking-related diseases; overall, smoking causes 1 in 5 deaths.25 Cigarette smoking is also associated with a high risk of intermittent claudication.26 Smokers develop symptoms of PAD almost a decade earlier than nonsmokers.6 Moreover, cigarette smoking is a stronger risk factor for PAD than for CAD.27 Smokers have been found to have more severe claudication pain, lower oxygen uptake, and higher ventilation compared with ex-smokers.28 This decrement confers greater risk of living a functionally limited lifestyle. In a case-control study evaluating the relationship of smoking, hypertension, heart disease, BMI, and high-density lipoprotein with PAD, cigarette smoking was found to be the most important risk factor for PAD.29 Patients with PAD who continue to smoke have a much higher risk of progressing to critical limb ischemia and are twice as likely to develop complications requiring amputation.6 Smokers with lower extremity bypass are three times (OR, 3.09; 95% CI, 2.34-4.08) more likely than nonsmokers to experience graft failure. Tobacco cessation is associated with survival benefit among claudicating patients.30 Nicotine is the addictive substance in tobacco and the primary factor associated with continued and compulsive tobacco use.31 Eight of every 10 patients who attempt to quit on their own return to smoking within a month, and each year, only 3% of smokers quit successfully.25 This underscores the importance of appropriate medical counseling and assistance. Regimented physician counseling programs have modest success rates of up to 6% at 1-year follow-up. In a systematic review of the efficacy of smoking interventions, it was found that personal advice and encouragement by physicians resulted in an estimated 2% of smokers quitting smoking for at least 1 year.32 Intensive multifaceted intervention for tobacco dependence is more effective for smoking cessation than single interventions.33 Other strategies for smoking cessation have been investigated, including nicotine patches and bupropion. In a meta-analysis of 2767 smokers in seven placebo-controlled trials, 6.75% of smokers receiving nicotine replacement therapy attained sustained abstinence for 6 months, twice the rate of those receiving placebo (relative risk [RR] 2.06; 95% CI, 1.34-3.15). These studies suggested that 29 patients would have to be treated to achieve 1 successful smoking cessation.34 In a double-blind, placebo-controlled comparison of sustained-release bupropion (244 subjects), a nicotine patch (244 subjects), bupropion and a nicotine patch (245 subjects), and placebo (160 subjects) for smoking cessation, the rate of continuous abstinence during the 12-month period was significantly higher in the three treated groups (16.4% nicotine patch, 30.3% bupropion, 35.5% bupropion and nicotine patch) than in the placebo group. Furthermore, the abstinence rate was significantly higher in the bupropion treatment groups than in the nicotine patch–alone group. Notably, over 30% of patients voluntarily withdrew from the study; most of them provided no specific information. Rates of discontinuation were 31.1% in the bupropion group, 35.7% in the nicotine patch group, and 28.6% in the combined-treatment group.35 Varenicline is a non-nicotinic partial agonist found to be superior to placebo for smoking cessation.31,36,37 This drug was developed by modifying the structure of the naturally occurring plant alkaloid cytisine, a partial agonist at the α4β2 nicotinic acetylcholine receptor that competitively blocks the receptor and only partially activates it.31 Varenicline leads to partial release of dopamine in the reward center at the same time it competitively inhibits receptor binding by the nicotine delivered from cigarettes, suppressing the symptoms of nicotine withdrawal and reducing the pharmacologic reward from cigarette smoking.31 Varenicline is more effective than sustained-release bupropion.37 In a randomized open-label trial with 746 patients, varenicline significantly reduced craving (P < 0.001), withdrawal symptoms (P < 0.001), and smoking satisfaction (P < 0.001) compared with nicotine replacement therapy.38 Varenicline at standard doses increases the chances of successful long-term smoking cessation between two- and threefold compared with pharmacologically unassisted cessation attempts.39 Combination therapy with varenicline and bupropion may be effective for increasing smoking abstinence rates above that observed with monotherapy. Similarly, combination therapy with varenicline and nicotine replacement therapy is safe and well tolerated.40,41 Despite the challenges, addressing smoking cessation remains the most important modifiable risk factor for prevention of vascular disease. Bupropion, nicotine replacement therapy, and varenicline appear cost-effective at preventing relapse in smokers attempting to quit or who have recently become abstinent; these medications have comparable cost-effectiveness to other nonpharmacologic smoking cessation interventions. Widespread use of these effective relapse prevention treatments would result in substantial health gain at an acceptable cost to patients and healthcare providers alike.42 Hypertension is an independent risk factor for development of PAD, and cardiovascular mortality may be reduced by its treatment.3,43 Approximately 2% to 5% of all patients with hypertension also have claudication.6 Conversely, in the Cardiovascular Health Study, more than half of those with an ABI less than 0.9 had hypertension.44 The current goals of therapy for hypertension in patients with PAD are to achieve a target blood pressure less than 140/90 mmHg in nondiabetics or less than 130/80 mmHg in patients with diabetes.3,45 However, the benefits of extremely tight blood pressure control among patients with diabetes have been recently challenged.46 In a study of 4733 participants with type 2 diabetes randomly assigned to intensive therapy (systolic pressure < 120 mmHg) or standard therapy (systolic pressure < 140 mmHg), intensive therapy did not reduce the composite rate of fatal and nonfatal major cardiovascular events.47 Initial therapies for management of hypertension include maintenance of ideal body weight, limited alcohol and sodium intake, cessation of smoking, and sufficient intake of potassium, magnesium, and calcium. However, most patients with known atherosclerosis will require pharmacologic intervention.
Management of Risk Factors for Peripheral Artery Disease
Introduction
Lifestyle Modification
Cigarette Smoking
Hypertension
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine