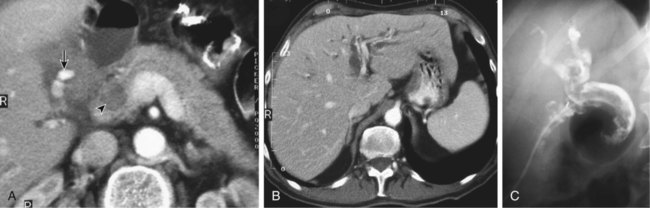
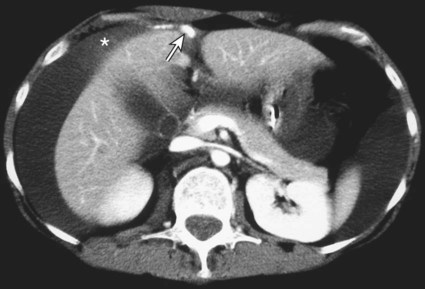
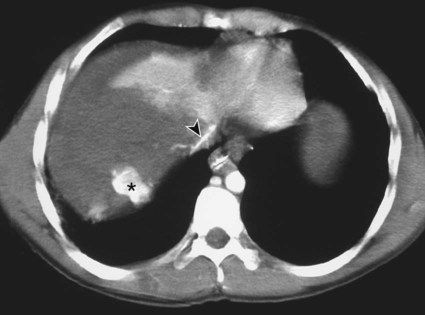
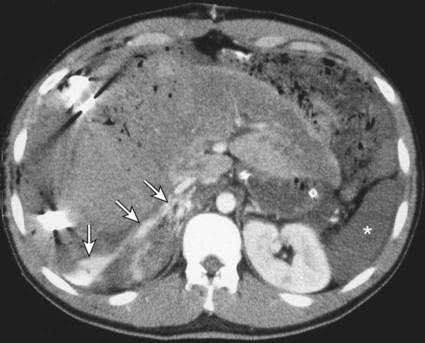
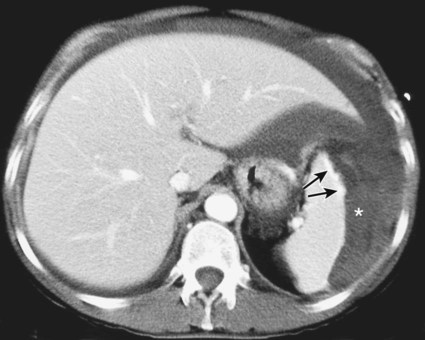
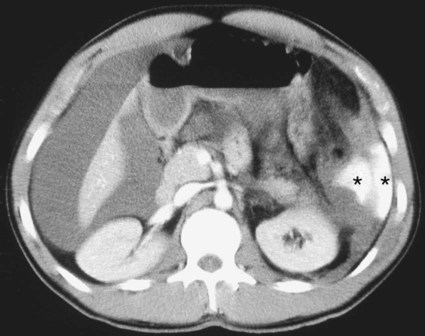
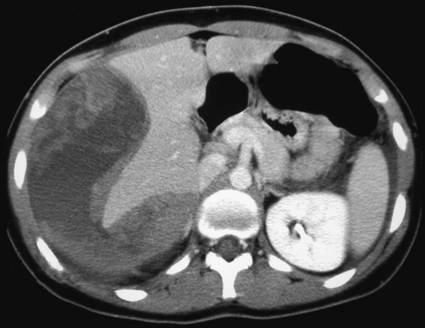
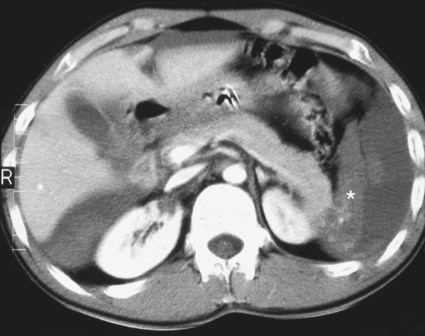
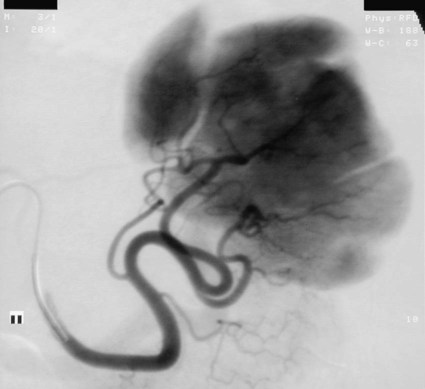
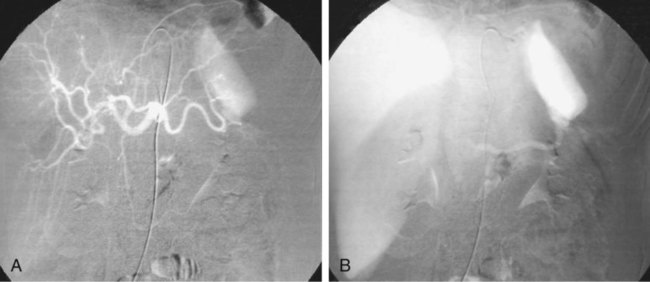
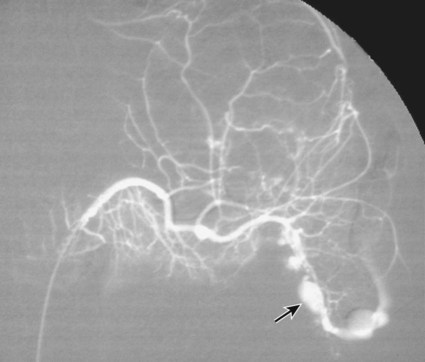
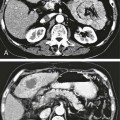
The diagnosis of hemobilia is indirectly established by endoscopy. Endoscopic retrograde cholangiopancreatography (ERCP) confirms bleeding from the papilla in only a minority of cases, but it shows intraluminal choledochal defects corresponding to clots, also occasionally seen in the pancreatic ducts, and provides evidence of proximal biliary obstruction (Fig. 71-1). ERCP does not show the intrahepatic bleeding site or the nature of the vascular lesion responsible for the hemobilia. Overall, ERCP is diagnostic of hemobilia in about a third of cases and can rule out a gastroduodenal bleeding source. Bile duct dilation often remains moderate because the clots are not totally obstructive. Endoscopic ultrasound (US) may be more contributory than ERCP by more precisely showing mobile hyperechoic material without acoustic shadows in the gallbladder and common bile duct, indicative of an endoluminal clot. Sphincterotomy with choledochal flushing removes clot from the bile ducts and exposes fragmented clot to the fibrinolytic activity of bile, thereby accelerating regression of the bile duct obstruction. Most often, the intrinsic fibrinolytic properties of bile cause clots to dissolve spontaneously. US, computed tomography (CT), and magnetic resonance imaging (MRI) can also suggest the diagnosis of hemobilia by showing echoic material in the bile ducts or hyperdense material and extra- and intrahepatic bile duct dilation and sometimes the intrahepatic vascular lesion. Blood pool scintigraphy may show accumulation at the site of an intrahepatic vascular lesion, but arteriography is definitive for both diagnosis and treatment. It locates the bleeding source in more than 90% of cases by demonstrating either an underlying vascular lesion—a pseudoaneurysm or an arterioportal fistula—or, in a minority of cases, extravasation of contrast material into the bile ducts. Spontaneous definite resolution of hemobilia without any type of intervention occurs in only a minority of patients. In a hemodynamically stable or rapidly stabilized patient after resuscitation, the observation on CT of dense extravasation of contrast material or a localized blush of contrast material within the liver or spleen parenchyma or diffusing through the capsule and diluting in the hemoperitoneum in the subphrenic, perihepatic, or perisplenic space is an unequivocal sign of ongoing bleeding or free intraperitoneal hemorrhage (Figs. 71-2 to 71-6). Inside the liver or spleen parenchyma, contrast material may line the capsule or layer in a hematoma. Extravasated contrast material shows characteristic attenuation values of 80 to 130 Hounsfield units (HU) and might be surrounded by hematoma with lower density. The density of extravasated contrast material is similar to that measured in large opacified vessels. Clotted blood, in the absence of extravasation, has density values around 60 HU, and unclotted free intraperitoneal blood, not mixed with contrast material, has values of 35 to 45 HU (Fig. 71-7). Extravasation of contrast material usually appears rapidly at the arterial phase of opacification. Massive and rapid accumulation of contrast material in the perihepatic or perisplenic spaces without continuity from an intravisceral contusion indicates rupture of a hilar vessel. Rarely, extravasation appears only on delayed scans. There might be several pitfalls in recognizing extravasation of contrast material on CT. In the case of severe hypovolemic shock, the arteries contract as a result of the output of vasoconstrictive agents. Severe arterial vasoconstriction is called hypoperfusion complex on CT images and may simulate disruption of the splenic artery by decreased enhancement or complete absence of splenic enhancement (Fig. 71-8; also see Fig. 71-4). This syndrome occurs in 0.01% to 2% of cases of major abdominal trauma in children with hypovolemic shock and may lead to unnecessary laparotomy. The CT appearance is particularly puzzling when no splenic enhancement at all is seen, but other solid organs are normally enhanced. The liver with its dual vascular supply is not usually affected, but the pancreas can be affected as an isolated organ in the same way as the spleen. When vasoconstriction resolves, progressive splenic enlargement may be seen on repeat CT examination. A sudden increase in intrasplenic blood inflow in an injured vessel may be responsible for early rebleeding despite apparent initial hemostasis. Theoretic causes of erroneous interpretation of hepatic or splenic injury on angiography include a false-positive diagnosis suggested by a splenic fissure; a nonopacified splenic polar artery; early venous return in the spleen caused by massive injection of contrast medium; superimposition of the gastric fundus, pancreatic tail, accessory spleen, or left adrenal; and preexisting hepatic or splenic infarct (Fig. 71-9). Because of the large projection area of the liver, false-positive results on arteriography are rare. False-negative diagnoses are due mainly to minimal parenchymal injury. False-negative findings of persistent hemorrhage are estimated to occur in 1% to 2% of patients. A multicenter survey found that arteriography is used today as a diagnostic modality in only 2% of patients being treated by observation. Angiographic access is gained by either a femoral or left axillary approach. Arteriography was used to evaluate hepatic or splenic injury in the past, but rapidly became obsolete with the advent of US and CT. For many years, arteriography was a reliable method for diagnosing subcapsular hematoma, large parenchymal contusion, posttraumatic vascular lesions, and extravasation of contrast medium. Typical arteriographic findings of hepatic or splenic injury include a parenchymal defect at the site of vascular interruption or contusion, a parenchymal blush of contrast medium, an arterioportal fistula, early venous return or displacement of intraparenchymal arteries, enlargement of the spleen, detachment of the liver or spleen from its contact with the diaphragm or the lateral abdominal wall, and spastic arterial contraction. In the liver, an avascular zone with marked displacement of vessels and dense parenchymal staining caused by compression usually corresponds to hematoma or biloma. Definitive angiographic signs are extravasation of contrast material or demonstration of a vascular injury (pseudoaneurysm or AVF) without extravasation of contrast material (Figs. 71-10 to 71-12).
Management of Trauma to the Liver and Spleen
Indications
Contraindications
Technique
Anatomy and Approach
Technical Aspects
Arteriographic Findings
Management of Trauma to the Liver and Spleen