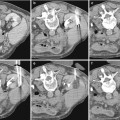
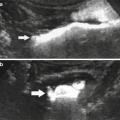
Fig. 1
X-ray picture of the abdomen 24 h after an angiographic procedure showing a persistent nephrogram. The patient experienced an acute renal failure following contrast administration
2.4 Risk Factors
Many large studies, most of them focusing on patients undergoing cardiac diagnostic and interventional procedures, considered the risk factors for CIN and contributed to enlarge our body of knowledge on this subject. We will consider risk factors that are related to the patient (most of them representing nonmodifiable patient characteristics) and those that are related to the procedure.
2.4.1 Patient-Related Risk Factors
The literature provides us a list of well-established risk factors for CIN, while some others are still questionable (Table 1).
Table 1
Patient-related risk factors for contrast-induced nephropathy (CIN)
Established | Questionable |
|---|---|
Preexisting renal impairment with DM | DM without renal impairment |
Preexisting renal impairment without DM | Hypertension |
Dehydration | Hyperuricemia |
Class III–IV congestive heart failure | Proteinuria |
Low left ventricular ejection fraction | Multiple myeloma |
Acute MI ≤24 h | Gender |
Intra-aortic balloon pump | Metabolic syndrome |
Periprocedural hypotension | |
Anemia | |
Old age | |
Administration of nephrotoxic drugs |
There is a general agreement that chronic kidney disease represents the most significant risk factor for CIN. Moreover, there is an association between the severity of the renal impairment and the risk of CIN (McCullough et al. 1997; Rihal et al. 2002). Furthermore, the association of chronic kidney disease and diabetes mellitus identifies the category of patients at the highest risk for CIN.
However, there is increasing evidence that patients referred to angiography should be considered at risk if their eGFR is <60 ml/min, while patients referred to CT should be considered at risk if their eGFR is <45 ml/min (see below).
Dehydration is normally listed among the risk factors, even if there is poor supporting evidence. Probably, dehydration was highly significant in the past when patients referred to intravenous urography experienced fluid restriction prior to the procedure. Congestive heart failure (New York Heart Association [NYHA] grades 3–4) turned out to be a predictor of CIN but only in studies considering patients undergoing cardiac catheterization. Such studies supported the significance of other risk factors, such as a left ventricular ejection fraction below 40 %, an acute myocardial infarction (within the last 24 h), and the use of an intra-aortic balloon pump, all features suggesting that a poor cardiac function is indeed a risk factor for CIN. The occurrence of hypotension during a vascular procedure (resulting in reduced blood supply to the kidneys) and anemia (resulting in poor oxygen supply to the kidneys) are other recognized risk factors. Old age (usually above 70 years) appears as an independent predictor of CIN according to some authors. An additional intuitive risk factor is the assumption of nephrotoxic drugs, although the supporting evidence is limited.
Questionable risk factors include diabetes mellitus in patients without renal impairment. Probably, diabetes is indeed an independent predictor for CIN, but there is no general agreement. Insulin-dependent diabetics appear to be at a higher risk.
Hyperuricemia is common in patients with chronic kidney disease and probably is not a risk factor per se. Female gender appeared as a risk factor according to some authors, but the majority of studies did not support this result. Hypertension and metabolic syndrome were recently considered significant risk factors. On the contrary, multiple myeloma was postulated to be extremely significant in the early literature, but a retrospective review and recent studies showed that this is not the case (McCarthy and Becker 1992; Pahade et al. 2011; Preda et al. 2011).
2.4.2 Procedure-Related Risk Factors
The risk of CIN is related to the contrast dose, and a number of studies showed that in patients at risk, the use of doses exceeding 100 mL for coronary angiography is associated with a higher rate of CIN. However, it should be reminded that even small volumes (20–30 mL) can cause CIN in patients at very high risk.
Laskey et al. (2007) estimated the maximum contrast volume to minimize CIN which should be equal to baseline CrCl × 3.7. A similar evaluation was performed by Nyman et al. (2008) who suggested limiting the contrast dose in grams of iodine numerically to the eGFR value in milliliter per minute.
The risk of CIN appears to be lower after intravenous vs. intra-arterial administration of contrast material (Katzberg and Lamba 2009). Of course, the route of administration is procedure specific, so there are no direct comparison trials, but a number of reasons may explain this difference, the main one probably being the lower volume of contrast which is usually injected in the intravenous procedures. Furthermore, lower concentration of contrast reaches the kidneys with intravenous administration; fewer patients with hemodynamic instability receive intravenous administration, and, finally, the catheter used in intra-arterial procedures can dislodge atheroemboli producing cholesterol embolization, with a resulting worsening of renal function that can mimic CIN.
After considering some papers on this issue (Katzberg and Lamba 2009; Kim et al. 2010; Weisbord et al. 2008), the Contrast Media Safety Committee of the ESUR concluded that patients referred for enhanced CT are genuinely at risk of CIN if they have an eGFR <45 ml/min (Stacul et al. 2011).
High osmolar contrast agents turned out to be more nephrotoxic than low osmolar agents in patients with impaired renal function following intra-arterial contrast administration (Barrett and Carlisle 1993). Comparative trials among low osmolar agents were limited and unable to detect significant differences with regard to renal safety. The relative nephrotoxicity of low osmolar and isoosmolar CM is a matter of debate since the publication of the NEPHRIC trial by Aspelin et al. (2003). This study showed that the isoosmolar nonionic dimer iodixanol was significantly less nephrotoxic than the low osmolar nonionic monomer iohexol in patients with renal impairment and diabetes who underwent angiography. A number of papers comparing iodixanol with different low osmolar agents were published since then. Heinrich et al. (2009) produced a meta-analysis of 25 randomized controlled trials (3,270 patients), 18 of them following intra-arterial and 7 of them following intravenous contrast injection, comparing iodixanol with nonionic monomers (iohexol, iopamidol, iopromide, iomeprol, ioversol, and iobitridol). They stated that iodixanol is not associated with a reduced risk of CIN after intravenous application. In patients with intra-arterial contrast application and renal insufficiency, the low osmolar agent iohexol is associated with a greater risk of CIN than is iodixanol, whereas nonsignificant difference between iodixanol and the other low osmolar agents could be found. This conclusion was additionally validated by more recent publications (Laskey et al. 2009; Wessely et al. 2009).
2.4.3 Coexistence of Multiple Risk Factors
The coexistence of multiple risk factors in a single patient can create a very high-risk scenario. It is extremely important to predict the actual risk in a single patient through adequate risk stratification. In the cardiology setting, three groups considered this topic and published the risk factor analyses (Bartholomew et al. 2004; Mehran et al. 2004; Brown et al. 2008). Multiple independent risk factors were identified and weighted. These analyses allowed creating a risk score for the prediction of CIN and showed that the risk of CIN increases exponentially with increasing risk score. Such a detailed analysis is unfortunately unavailable following intravenous contrast administration, in the CT setting, and is warranted.
Kim et al. (2011) developed a first risk stratification nomogram for CIN in CT considering 750 patients and considering age and baseline serum creatinine as risk factors.
2.5 Strategies for Risk Reduction
The first issue to be considered for preventing CIN is the identification of patients at risk, above all, patients with eGFR <60 mL/min/1.73 m2. Determination of eGFR (or serum creatinine) within 7 days of contrast medium administration is required in patients with known chronic kidney disease, in patients who will receive intra-arterial contrast medium, in patients over 70, and in patients with a history of renal disease, renal surgery, proteinuria, diabetes mellitus, hypertension, gout, and recent assumption of nephrotoxic drugs (Stacul et al. 2011).
In patients at risk for CIN, the possibility of performing another procedure not requiring the use of iodinated contrast agents (ultrasound, MRI) should be considered first. Of course, the issue of the possible onset of NSF in patients referred to contrast-enhanced MRI has to be carefully evaluated (see below). If the procedure requiring iodinated contrast is deemed necessary, a number of strategies to reduce the risk of CIN can be considered, namely, hydration, use of pharmacological agents, withdrawal of nephrotoxic drugs, hemodialysis and hemofiltration, choice of contrast material type, and dose.
2.5.1 Hydration Protocols
There is a general consensus that hydration is effective in preventing CIN, because it is effective in preventing the renal damage from other injuries even though randomized double-blind comparative trials considering hydration vs. no hydration are not available so far. It appears that intravenous hydration (more properly, volume expansion) is more effective than oral hydration (Trivedi et al. 2003), but the evidence is poor and data on the possible efficacy of oral hydration are lacking. Intravenous administration of normal (0.9 %) saline is more effective than half-normal (0.45 %) saline (Mueller et al. 2002). Most studies in the cardiology setting suggest administering 1.0–1.5 mL/kg/h starting 12 h before the procedure, till 12 h after the procedure. However, this regimen is still impractical in the outpatient setting and trials considering the best timing for hydration are highly advisable.
It is worthwhile mentioning the possibility of performing the volume expansion using sodium bicarbonate instead of sodium chloride with the rationale of producing an urine alkalinization that could theoretically prevent the formation of oxygen free radicals with the resulting renal damage. Following the first results favoring bicarbonate (Merten et al. 2004), a large number of studies and meta-analyses were published. The meta-analyses show a certain degree of heterogeneity and publication bias, but it seems that the evidence in favor of bicarbonate is now pretty strong. Additionally, it is worthwhile mentioning that administration of bicarbonate is suggested to start 1 h prior to the procedure till 6 h after, a more practical regimen for outpatients.
Therefore the Contrast Media Safety Committee of the ESUR (Stacul et al. 2011) advocates either the administration of 1 mL/Kg/h of normal saline for at least 6 h before and after the procedure or the administration of sodium bicarbonate (3 mL/Kg/h for 1 h before contrast medium followed by 1 mL/Kg/h for 6 h after).
2.5.2 Pharmacological Agents and Withdrawal of Nephrotoxic Drugs
A pharmacological prophylaxis for preventing CIN would be advisable, and a large number of drugs were tested for this purpose. A review (Stacul et al. 2006) subdivided these drugs into three groups: potentially beneficial agents that need further evaluation but could be considered for use in patients at risk (theophylline/aminophylline, statins, ascorbic acid, prostaglandin E1), agents that have not been shown to be consistently effective in reducing the risk of CIN (N-acetylcysteine (NAC), fenoldopam/dopamine, calcium-channel blockers, atrial natriuretic peptide), and potentially detrimental agents (furosemide, mannitol, endothelin receptor antagonist). Among them, NAC is more widely used (with a standard oral regimen of 600 mg twice daily the day before and on the day of the procedure), given its low cost, safety (when administered orally), and wide availability. Nevertheless, its efficacy is still questionable despite a large number of trials and meta-analyses. The largest trial (Webb et al. 2004) failed to show a benefit of this drug, while the last published meta-analyses (Kelly et al. 2008) favors its use.
The Contrast Media Safety Committee of the ESUR, given the heterogeneous results of trials and meta-analyses, does not recommend, for the time being, any pharmacological manipulation for routine use in the prevention of CIN (Stacul et al. 2011).
It is reasonable to assume that nephrotoxic drugs increase the risk of CIN, although this topic was poorly addressed in the literature. Alamartine et al. (2003) reported a higher incidence of CIN in patients receiving anti-inflammatory drugs, aminoglycosides and amphotericin B. Other drugs, such as cyclosporine, tacrolimus, and cisplatinum appear to increase the risk. Withdrawal of nephrotoxic drugs at least 24 h before the procedure is therefore suggested, when clinically compatible, and should be discussed with the referring physician, balancing relative benefits and harms.
2.5.3 Hemodialysis and Hemofiltration
Hemodialysis is actually effective in removing contrast material, but ineffective in preventing CIN even when carried out immediately after the contrast-enhanced procedure. These results are consistent with the theory that the renal damage eventually occurs very rapidly after contrast administration, probably mainly related to the contrast material-induced vasoconstriction. Therefore, hemodialysis is not recommended for preventing CIN.
Marenzi et al. (2003, 2006) reported the favorable results of performing hemofiltration (started 4–8 h before percutaneous coronary intervention and continued for 18–24 h afterward) for preventing CIN. Of course, hemofiltration itself affects SCr levels, and therefore, SCr reduction may be unrelated to renal function, but it is worthwhile mentioning that inhospital and 1-year mortality significantly improved in the hemofiltration group. However, the high cost of the procedure and the need for intensive care unit admission limits its utility.
2.5.4 Choice of Contrast Material Type and Dose
This issue was already considered when procedure-related risk factors were discussed (see Sect. 2.4.2). When considering the renal safety, there are no arguments favoring the administration of one or another low or isoosmolar agent through the intravenous route. When intra-arterial studies are planned, the present evidence suggests that iodixanol is less nephrotoxic than the nonionic monomer iohexol and the ionic dimer ioxaglate in patients with impaired renal function. Other comparative evaluations were unable to detect significant differences among the products.
The contrast dose to be injected should always be the lowest compatible with a diagnostic result. Recent trials (Laskey et al. 2007; Nyman et al. 2008) provided valuable information on the maximum dose of contrast to minimize the risk of CIN.
When considering contrast material injection in patients at risk for CIN, planning of multiple studies is an issue. Unfortunately, clinical trials providing suggestions on the optimal timing between two consecutive contrast-enhanced studies are not available, but waiting for 2 weeks, the expected recovery time of the kidney following an acute injury, appears reasonable, if compatible with the diagnostic requirements.
2.6 Open Questions
Despite the large number of studies that were published in the last years on this topic, many questions concerning CIN are still open and some new problems arose.
Studies by Newhouse et al. (2008) and Bruce et al. (2009) underlined that physiologic variations in SCr levels may fulfill the CIN definitions. Bruce et al. (2009) identified a high incidence of acute kidney injury among control subjects undergoing unenhanced CT and suggested that the additional risk of acute kidney injury accompanying the administration of contrast medium may be overstated and much of the creatinine elevation in these patients is attributable to the background fluctuation, underlying disease, or treatment. McDonald et al (2013) considered 167,140 enhanced and unenhanced scans in 53,439 patients and concluded that intravenously injected contrast media may not be the cause of reduced renal function after contrast administration. Davenport et al. (2013) performed a similar analysis and showed that intravenously injected contrast medium is a nephrotoxic risk factor. These results have significant implications in the planning of future trials considering CIN that should eventually consider one control arm as well, but optimal randomization appears extremely difficult (Thomsen et al. 2013a).
The production of validated risk scores for patients undergoing intra-arterial studies underscores the need for a similar model for predicting the risk of CIN in a single patient referred to intravenous contrast administration. Patients might be stratified according to the different risks and it appears reasonable that patients at different risks may require different prevention protocols.
An effort to produce different protocols is advisable and will favorably affect the management of at-risk patients in daily practice. Such practice will benefit from additional data on optimal timing and duration of hydration. More data on the potential benefit of sodium bicarbonate and NAC are advisable as well. Furthermore, larger multicenter trials in which clinically relevant outcomes are assessed are required to definitely resolve the issue on whether the nephrotoxicity of iodixanol is less than that of some of the low osmolar agents after intra-arterial application in high-risk patients, as Heinrich et al. (2009) pointed out.
2.7 Guidelines
The Contrast Media Safety Committee of the ESUR produced in 1999 a guideline for preventing CIN (Morcos et al. 1999) which was recently updated (Stacul et al. 2011) and is available at www.esur.org. It is interesting to realize that after 15 years and despite a huge number of studies and reviews of CIN, nothing changed substantially. Thomsen et al. (2008b) underlined that the strategies for CIN prevention are still use of the smallest possible dose of low – or isoosmolar contrast agent, volume expansion, withdrawal of nephrotoxic drugs, and avoidance of repeat contrast injections within 48 h.
Additionally, both guidelines by the American College of Radiology (ACR Manual Contrast Media, 2013), available at www.acr.org, and by nephrologic societies (Fliser et al. 2012) provide suggestions that are very close to ESUR recommendations, thus further validating these advises.
3 Nephrogenic Systemic Fibrosis
3.1 Definition
NSF is a severe systemic fibrosing disorder associated with the exposure to gadolinium-containing CM. The first case was identified in 1997 and the first report in the peer-reviewed literature occurred in 2000. At the beginning, the disease was thought to involve only the skin and was termed nephrogenic fibrosing dermopathy. Later, it became apparent that multiple organs could be affected (Cowper 2005), and in 2006, a relationship between the disease and the administration of Gd-containing CM was suggested for the first time (Grobner 2006; Marckmann et al. 2006).
3.2 Clinical Features
The clinical features vary from one patient to another and over time. However, the large majority of the patients had their initial NSF symptoms within 2 months following Gd-containing CM exposure, although this interval is reported to vary to a large extent. The early symptoms include pain, itching, erythema, and swelling. Skin changes primarily involve the lower limbs. Late symptoms include skin thickening and hardening, and the affected area may have a peau d’orange appearance and a woody texture (Fig. 2). The involvement of other organs is frequent (neuropathic symptoms, respiratory insufficiency caused by lung fibrosis, muscular atrophy, etc.). Joint contractures (Fig. 3), leading to disabilities and dependence on wheelchair, occur in more severe cases (Cowper 2008) and death is reported in up to 5 % of the patients.
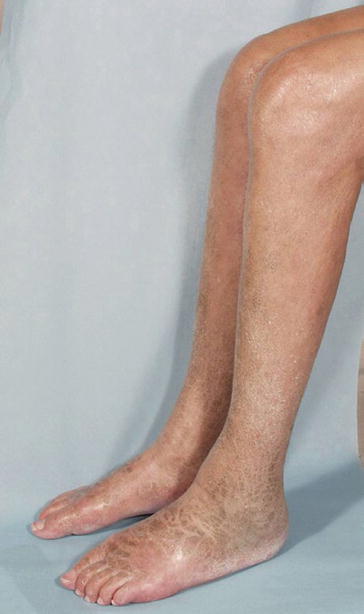
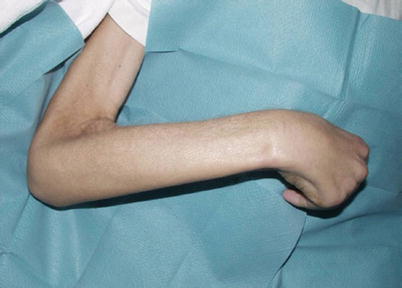

Fig. 2
A 35-year-old female patient on hemodialysis suffering from nephrogenic systemic fibrosis (NSF): discolored skin plaques and atrophy of the calf muscles are noticeable (Courtesy of Prof. H. Thomsen)

Fig. 3
A 49-year-old male patient with CKD 5 suffering from NSF: muscular atrophy and contractures are clearly depicted (Courtesy of Prof. H. Thomsen)
Most patients who experienced NSF are middle aged, but the age range is very large (8–87 years), without ethnicity or gender predilections.
3.3 Risk Factors
Two factors appear to coexist in the history of patients who developed NSF: the impaired renal function and the administration of Gd-containing CM (Agarwal et al. 2009; Broome et al. 2007; Sadowski et al. 2007; Van der Molen 2008). Patients with chronic kidney disease stage 4 and 5 (GFR <30 mL/min/1.73 m2) appeared to be at high risk for NSF, the large majority of them being included in stage 5. Hemodialysis patients appear to be at risk for NSF in its most severe form, more than peritoneal dialysis patients.
Patients with chronic kidney disease stage 3 (GFR 30–59 mL/min/1.73 m2) are to be considered at risk to a lower extent. It is extremely significant that not a single case of NSF was reported among patients with GFR >60 mL/min/1.73 m2.
Recent data suggest that there is no increased risk of NSF in patients with liver dysfunction but normal renal function (Chow et al. 2011). There have been no published reports of NSF in neonates, but their immature renal function suggests they may be at risk if low stability gadolinium agents are injected.
Almost all NSF patients received Gd-containing CM in their medical history. Actually, Broome (2008) reviewed the medical literature and reported that only five patients had NSF without previous exposure to Gd-containing CM. Deng et al. (2010) collected seven NSF patients who apparently did not receive Gd-containing agents, but in three of them, high levels of gadolinium were detected in the skin, suggesting a previous unreported exposure to the agent.
Furthermore, because NSF does not occur in all patients with impaired renal function injected with gadolinium chelates, it was suggested that other cofactors may be involved in the pathogenesis of this disease, such as proinflammatory events (surgical procedures – particularly vascular interventions and liver transplantation, severe sepsis, hypercoagulability), metabolic acidosis, high levels of erythropoietin, and immunologic disease. However, the hypothetical role of cofactors is still awaiting confirmation.
3.4 The Role of Contrast Material
Nine Gd-containing CM are approved for clinical use.
When considering the market share in the 1997–2006 period, gadopentetate dimeglumine (Magnevist®) was the leader, being used in more than 61,000,000 procedures, followed by gadodiamide (Omniscan®) in more than 33,000,000, gadoteridol (ProHance®) in more than 10,000,000, gadobenate dimeglumine (MultiHance®), and gadoversetamide (OptiMARK®). However, when considering cases of NSF, the distribution is not the same: Broome (2008) collected 195 cases published on peer-reviewed journals and bioptically confirmed and showed that the large majority of them (157 out of 195) received gadodiamide, eight of them received gadopentetate dimeglumine, and three received gadoversetamide. The contrast agent was unknown in 18 patients, while four received multiple agents, and in five of them no Gd-containing agent was registered in the medical history. Besides the medical literature, different databases on NSF are available (International Center for NSF Research, FDA, regulatory authorities of different European countries, Contrast Media Committee of ACR, ESUR) and data can differ largely. Heinz Peer (2009




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree