Mediastinal and Chest Wall Masses
Teresa Chapman, MD
LEARNING OBJECTIVES
1. Localize masses to either the mediastinum or the chest wall, based on radiographic margins and osseous changes.
2. List appropriate diagnostic considerations for a mediastinal mass in the child.
3. Differentiate Hodgkin lymphoma from non-Hodgkin lymphoma, based on features such as involved nodal station contiguity, and involvement of pericardium and pleura.
4. Identify appropriate serum markers when considering a mediastinal germ cell tumor.
5. Differentiate thymoma from thymic hyperplasia based on CT findings.
6. Recognize differences in tumor morphology and calcification patterns seen in intrathoracic neuroblastoma, ganglioneuroblastoma, and ganglioneuroma.
7. Provide differential diagnosis for an aggressive chest wall mass in the child, and indicate which tumor type typically invades through the chest wall.
INTRODUCTION
Pediatric masses of the mediastinum and the chest wall encompass a wide range of histopathologies, and constitute a significant percentage of oncologic and cardiothoracic surgical cases. Clinical presentations vary from palpable abnormalities to constitutional symptoms to significant respiratory distress, and in other cases, these masses are incidentally found on imaging. The mediastinum is defined anatomically by the parietal pleura laterally, the thoracic inlet superiorly, the diaphragm inferiorly, the sternum anteriorly, and the spine posteriorly. Definitions of mediastinal compartmentalization differ between anatomists, surgeons, and radiologists. Whereas anatomists might distinguish the superior mediastinum (the space above the pericardium, extending to the thoracic inlet) from the anterior and middle mediastinum, radiologists tend to merge the superior mediastinum with the anterior and middle mediastinum.1 The anterior mediastinum is anterior to the pericardium and contains the retrosternal space and the thymus. The middle mediastinum contains the pericardium and it contents, the ascending great arteries, the superior vena cava, the trachea and mainstem bronchi, the pulmonary arteries, and bronchial lymphatics. The posterior mediastinum is posterior to the pericardium and contains the esophagus, the descending thoracic aorta, the azygous and hemiazygos veins, the thoracic duct, and lymph nodes. The chest wall includes the pleura, the bony thorax, and its supporting muscles. Tumor types, therefore, may arise from any of the tissues in these compartments. In this chapter, the patient demographics, imaging findings, and management of these tumors are discussed and reviewed.
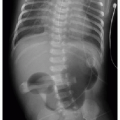
APPROACH TO DIAGNOSIS
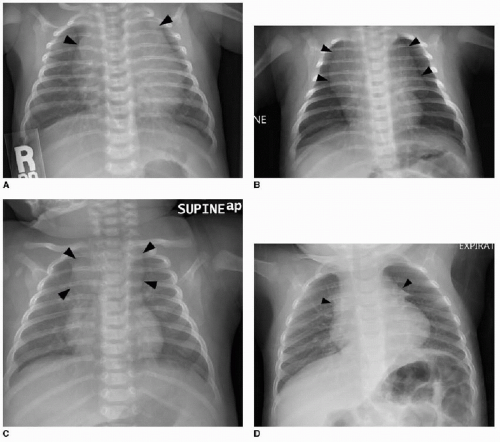
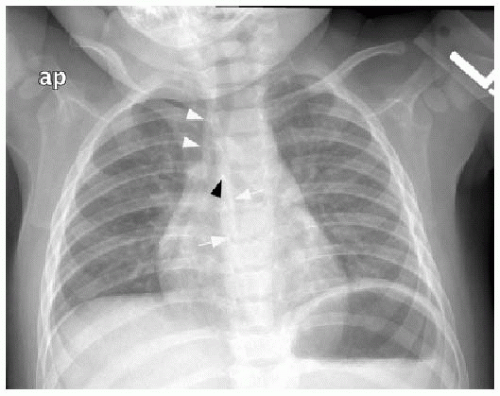
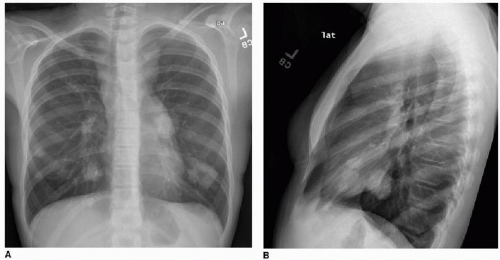
This chapter first discusses the radiologist’s approach to recognizing the presence of a nonpulmonary mass in the chest. Regardless of the patient’s age or presentation, evaluation of the chest radiograph should follow a systematic approach. Any given individual survey of the chest radiograph can successfully lead to recognition of a chest mass, as long as mediastinal contours and the bones are carefully inspected. Interpretation of cardiomediastinal contours can be difficult in infants and toddlers because the thymus gland can be quite generous in that age group (Fig. 6.1). The thymus gland on radiography has partially well-defined margins and is not so dense that it obscures pulmonary interstitial markings, as opposed to mediastinal masses, that usually are dense enough to obscure lung and often have well-defined and lobulated margins. The thymus gland typically decreases in size gradually over the first several years of life, and the superior mediastinal contours after age 2 to 3 years tend to be narrower. In the older child and adolescent, assessment of the mediastinal contours includes attention to the paratracheal density, which should be uniformly thin, and to the azygoesophageal recess and subcarinal space, which should
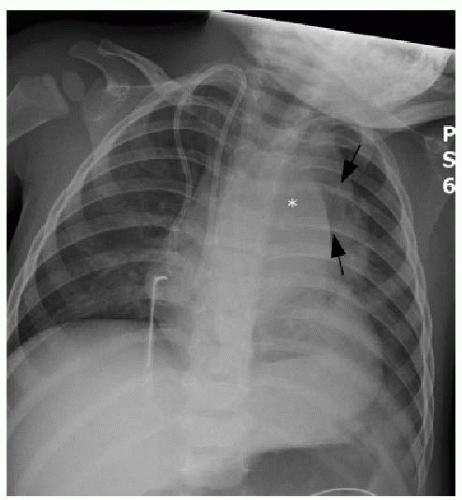
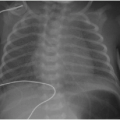
be clear (Fig. 6.2). Posterior mediastinal masses, specifically neurogenic tumors, and chest wall masses may be less obvious than the changes in rib position, morphology, or density that they induce. On every pediatric chest radiograph, be careful to assess the posterior ribs for widening (Fig. 6.3) or destructive changes.
be clear (Fig. 6.2). Posterior mediastinal masses, specifically neurogenic tumors, and chest wall masses may be less obvious than the changes in rib position, morphology, or density that they induce. On every pediatric chest radiograph, be careful to assess the posterior ribs for widening (Fig. 6.3) or destructive changes.
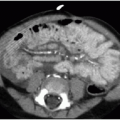
Further assessment of a suspected mediastinal or chest wall mass based on radiography may be performed using either contrast-enhanced CT or thoracic MR imaging.2,3 These cross-sectional techniques will confirm the presence of a mass, establish location and involvement of thoracic structures, and may offer insight into the likely pathology, based on shape, margins, density, or signal characteristics. Lower apparent diffusion coefficiency within mediastinal masses has been shown to be associated with malignant tissue types.4 When imaging characteristics
do not definitively indicate the tumor type, a biopsy may be considered for tissue diagnosis. Prior to this, however, the impact of any mass upon the airways must be carefully assessed. Airway occlusion is a well-recognized complication of general anesthesia in children with anterior mediastinal masses, and a diagnostic radiologist should understand this before suggesting a percutaneous biopsy be performed for a tissue diagnosis. Preprocedure prognostic indications of at-risk patients include a tracheal crosssectional area less than 50% expected or a peak expiratory flow rate less than 50% of predicted for age and gender.5 In certain cases, management with steroids or radiation therapy may be required before a tissue diagnosis is possible. Less invasive means of diagnosis, such as pleural aspiration and bone marrow biopsy, may also be possible.6
ANTERIOR MEDIASTINAL MASS: LYMPHOMA
In the pediatric patient, across all ages, the most commonly diagnosed malignant anterior mediastinal mass is lymphoma.7 This category of disease can be divided into Hodgkin lymphoma and non-Hodgkin lymphoma (NHL).
Hodgkin lymphoma constitutes 6% of childhood cancers, and the incidence is highest in adolescents aged 15 to 19 years.8 Of all adolescent and young adult cases of Hodgkin disease, 75% of these present with mediastinal involvement; in contrast, only 35% of younger children with Hodgkin lymphoma have a mediastinal presentation.9 Ebstein-Barr virus is associated with Hodgkin lymphoma, most strongly in the mixed cellularity tumors and in patients less than 10 years of age.10 The prognosis for individuals with Hodgkin lymphoma is good, with a greater than 90% 5-year survival rate. Hodgkin lymphoma is staged according to extent of disease: Stage I is localized to a single nodal site; Stage II involves two or more nodal stations on the same side of the diaphragm; Stage III involves disease on both sides of the diaphragm; and Stage IV involves diffuse disease, including spread to the liver, lungs, bone marrow, or cerebrospinal fluid.
NHL accounts for approximately 7% of cancers in children younger than 20 years.8,11 NHL is rare in infants. NHL includes four subtypes: Burkitt lymphoma, diffuse large B-cell lymphoma, anaplastic large cell lymphoma, and lymphoblastic lymphoma. Mediastinal involvement by NHL in children and adolescents results in poorer outcomes than does mediastinal NHL in adults: the 3-year event-free survival rate in children and adolescents with mediastinal B-cell lymphoma is only 50% to 70%.12,13 Any intrathoracic NHL tumor in a pediatric patient is at least Stage III disease (Stage IV disease includes cases with bone marrow or CNS involvement). Of note, T-cell leukemia may present with a mediastinal mass and pleural effusions, and the differentiation of this from lymphoblastic lymphoma is made arbitrarily by the bone marrow involvement (if greater than 25% blasts are present in the marrow, the diagnosis is leukemia).14
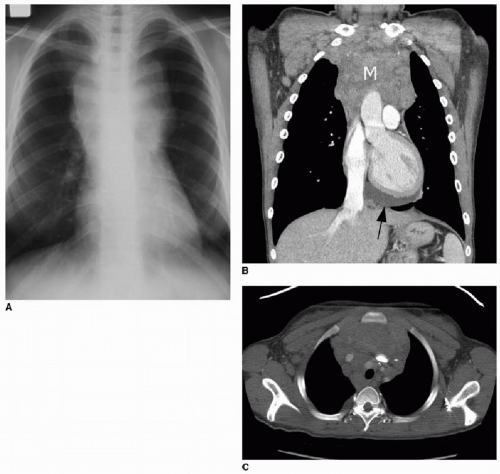
Clinical presenting signs of mediastinal lymphoma overlap with other mediastinal masses and may include respiratory difficulty, cough, chest pain, fever, and night sweats. Cross-sectional imaging of the thorax will typically show a large multilobulated mass that reflects the infiltrated thymus gland, in addition to adjacent involved lymph nodes. Direct invasion of the pericardium, which occurs most often with Hodgkin lymphoma, causes a pericardial effusion (Fig. 6.4).7 NHL may invade the chest wall. Associated pulmonary nodules and pleural effusions (Fig. 6.5) only occur in approximately 5% of Hodgkin lymphoma cases, but are seen in up to 75% of NHL cases.15
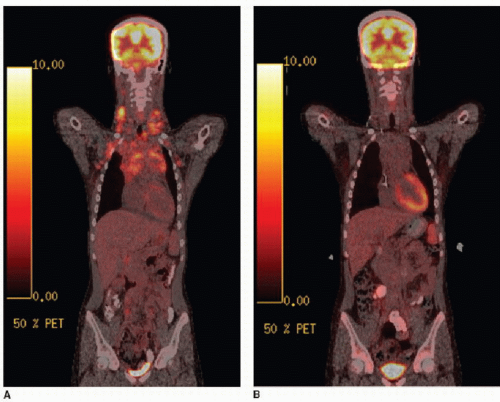
Staging of lymphoma is completed by chest radiography, CT of the neck, chest, abdomen, and pelvis, and by 18F-FDG positron emission tomography (PET) imaging (ideally with CT fusion; see Fig. 6.6). The definition of abnormal lymph node enlargement by CT criteria is most commonly accepted as greater than 15 mm in long-axis diameter and greater than 10 mm in short-axis diameter. Protocols for treatment vary, however, and some protocols may consider nodes greater than 20 mm in largest diameter as involved by disease and nodes less than 10 mm in largest diameter as disease free, with additional clinical and laboratory information used for equivocal imaging.16 In general, areas of FDG positivity that do not correspond to an anatomic lesion by clinical or diagnostic CT examination should be disregarded in staging. Furthermore, a suspected lesion identified clinically or identified by CT and subsequently found to be FDG-negative should not be considered involved by disease, unless proven by biopsy.
The rapidity of response to the initial cycles of chemotherapy is an important prognostic factor. This is assessed qualitatively and quantitatively by cross-sectional imaging as well
as by FDG-PET (Fig. 6.6). FDG avidity after the first cycle of chemotherapy in children has been shown as a useful measure to predict progression-free survival,17,18 and FDG avidity in children after initial chemotherapy cycles can predict diseasefree survival and guide radiation therapy planning.19 Thymic rebound enlargement is a well-known phenomenon following chemotherapy,20 and this can be confusing on follow-up staging studies of mediastinal lymphoma. FDG-PET has been shown to be useful here, as well. A standard uptake value (SUV) of 3.4 or higher has been shown to be a strong predictor of mediastinal lymphoma.21
as by FDG-PET (Fig. 6.6). FDG avidity after the first cycle of chemotherapy in children has been shown as a useful measure to predict progression-free survival,17,18 and FDG avidity in children after initial chemotherapy cycles can predict diseasefree survival and guide radiation therapy planning.19 Thymic rebound enlargement is a well-known phenomenon following chemotherapy,20 and this can be confusing on follow-up staging studies of mediastinal lymphoma. FDG-PET has been shown to be useful here, as well. A standard uptake value (SUV) of 3.4 or higher has been shown to be a strong predictor of mediastinal lymphoma.21
ANTERIOR MEDIASTINAL MASS: GERM CELL TUMOR
Extragonadal germ cell tumors (GCTs) are rare and are more commonly seen in males than in females. They can primarily arise anywhere, although midline sites (such as the mediastinum, retroperitoneum, and pineal gland) are the most common sites of origin.22 Primary mediastinal GCTs account for up to 18% of pediatric mediastinal masses, and 60% to 86% of these are benign.2,23,24 The benign types are the mature and immature teratomas. Malignant GCT often contains coexisting benign components; aggressive tissue types seen in the pediatric population include seminomas (germinomas), endodermal sinus tumor, yolk sac tumor, and choriocarcinoma. Seminomas typically present in males in the second or third decade and are therefore differential considerations in adolescents.
Mediastinal GCTs tend to arise within or near the thymus but may also arise within the middle or posterior mediastinum.25 Malignant tumors tend to be large at presentation with substantial mass effect upon the intrathoracic structures, causing severe symptoms. Clinical presenting signs are age-dependent: in the fetus, a mediastinal teratoma can be identified by prenatal ultrasound as a well-circumscribed mediastinal mass usually associated with hydrops26,27; infants and younger children present with
respiratory symptoms; older patients present with chest pain, cough, fever, or precocious puberty.24 There is a well-recognized association of Klinefelter syndrome with nonseminomatous GCT.24 Laboratory evaluation may indicate cellular components of these tumors: alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (bHCG), and lactate dehydrogenase (LDH) may be elevated in the nonseminomatous malignant GCTs, including malignant teratoma.28,29
respiratory symptoms; older patients present with chest pain, cough, fever, or precocious puberty.24 There is a well-recognized association of Klinefelter syndrome with nonseminomatous GCT.24 Laboratory evaluation may indicate cellular components of these tumors: alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (bHCG), and lactate dehydrogenase (LDH) may be elevated in the nonseminomatous malignant GCTs, including malignant teratoma.28,29
The appearance of mediastinal teratomas and other GCTs on CT and MR studies varies, depending on the tissue content. The teratoma is usually a well-defined cystic mass containing fluid, soft tissue, calcification, and fat (Fig. 6.7). Fat-fluid levels within a mediastinal tumor are diagnostic of a teratoma. Rupture into adjacent structures (airways, lung, mediastinum, and pleural spaces) may occur and may be secondary to secretion of pancreatic or intestinal enzymes in mature tumors.2 Seminomas (germinomas) tend to be well-demarcated solid masses with internal heterogeneous densities on CT and signal changes on MR that reflect hemorrhage and necrosis. Nonseminomatous malignant GCTs are also heterogeneous (Fig. 6.8) and may show ill-defined margins due to invasion of adjacent structures.30
Malignant tumors carry a poor prognosis, although survival rates have improved with platinum-based combination chemotherapy protocols.31




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree