Mediastinum
Bernard F. Laya
Mark C. Liszewski
Evan J. Zucker
Ricardo Restrepo
Edward Y. Lee
INTRODUCTION
The mediastinum is located in the central chest between the right and left pleural cavities and spans from the thoracic inlet to the diaphragm. This compartment contains vital structures of the circulatory, respiratory, digestive, and nervous systems. The mediastinum is the most common location for nonvascular masses of the chest in the pediatric population.1 These masses, which represent congenital lesions, infections, and benign or malignant neoplasms, have a wide spectrum of radiological and histopathological manifestations. There are also myriad vascular mediastinal lesions, which may be either congenital or acquired and involve the systemic and pulmonary arteries and veins in various combinations.
Imaging plays an important role in detecting and characterizing mediastinal masses in order to optimally direct patient management. Thus, a practical and systematic imaging approach is important. This chapter begins with a description of the embryological development and radiologic anatomy of the mediastinum. For standardization, the CT-based compartment scheme for the mediastinum that was introduced by the International Thymic Malignancy Interest Group (ITMIG) is utilized in this chapter.1,2 Various currently available imaging techniques for the evaluation of mediastinal lesions are presented, along with suggestions for a practical approach to imaging. The wide spectrum of nonvascular and vascular lesions is discussed, with descriptions of typical imaging manifestations and current management considerations pertaining to relevant disorders in the pediatric population.
ANATOMY
Embryology
Specific structures in the mediastinum develop at different stages of embryonic life. The intraembryonic coelom, the primordium of the body cavities, develops by the end of the 3rd week. In the 4th gestational week, this horseshoe-shaped primordium gives rise to three well-defined body cavities lined with mesodermal mesothelium that ultimately form the boundaries of the mediastinum. These are the pericardial cavity, peritoneal cavity, and two pericardioperitoneal canals that interconnect them (Fig. 3.1A). During this time, there is also formation of partitions that separate the pericardium from the pleural cavities and the peritoneum (Fig. 3.1B).3,4 The parietal layer of mesoderm lining these cavities becomes the parietal peritoneum, parietal pleura, and serous pericardium.3
During the 7th week of gestation, the pleuropericardial membranes fuse with the mesoderm ventral to the esophagus, forming a defined area called the primitive mediastinum, which is distinct from the pleural cavities (Fig. 3.1C). The pleuropericardial canals are situated at the lateral borders of the proximal foregut, which divides into the esophagus and tracheal buds. The eventual growth of the bronchial buds (primordia of the bronchi and lungs) pushes the superior pleuropericardial surfaces upward and the inferior surfaces downward, defining the pleural cavities and creating space for the mediastinal structures. This primordial mediastinum consists of a mass of mesenchyme that extends from the sternum to the vertebral column, separating the developing lungs.3,4
The diaphragm develops from the septum transversum, mesentery of the esophagus, pleuroperitoneal folds and membranes, and muscular outgrowth of the body wall. Fusion of the caudal pleuroperitoneal membranes during formation of the diaphragm separates the pleural and peritoneal cavities.3
Cardiac development is noted as early as the 3rd week of gestation, with cardiac contractions detected at 21 or 22 days of gestation. Approximately 2 weeks later, there is rotation of the primitive atrioventricular canal that transforms the tubular heart into a four-chambered organ (Fig. 3.1B and C).4
The thymus, which produces hormones that stimulate the maturation of T cells, is the first lymphoid organ to be formed. It arises from the third pharyngeal pouch but may also contain tissue from the fourth pharyngeal pouch. The thymic tissue elongates during the 7th gestational week, and each lobe migrates inferiorly and into the midline by the 8th week. Although the right and left primordial thymic lobes are distinct, they are closely apposed in midline, with a small bridge of connecting tissue. For the remainder of gestation, the thymus continues to grow.5,6
Brief descriptions of the embryologic development of other specific mediastinal organs, as they pertain to various pathologies, are discussed in the following sections.
Normal Development and Anatomy
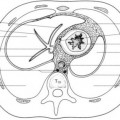
The mediastinum is an intricate thoracic compartment containing vital structures such as the heart and great vessels, trachea and main bronchi, esophagus, thymus, veins, lymphatic structures, and nerve tissue. It is a movable partition that is bound superiorly by the thoracic inlet, inferiorly by the diaphragm, laterally by the parietal pleural reflections along the medial aspects of both lungs, anteriorly by the sternum, and posteriorly by the anterior surfaces of the thoracic vertebral bodies (Fig. 3.2).7
In generating differential diagnoses, it is important to use a standardized method for classifying the mediastinal compartments. Several classification systems that have been developed by anatomists, clinicians, and radiologists include the Fraser and Paré, Felson, Heitzman, Zylak, and Whitten methods.8,9,10,11,12 However, these methods use differing terminology and classification schemes that sometimes lead to confusion.2,13,14 The compartmentalization scheme developed by Felson, which divides the mediastinum into three divisions, was based on landmarks on lateral chest radiography, and sometimes cannot accurately distinguish lesion location.8 Currently, investigation of mediastinal abnormalities with cross-sectional imaging (either computed tomography [CT] or magnetic resonance imaging [MRI]) is the gold standard; therefore, it is sensible to use a classification strategy that pertains to these modalities.
The Japan Association for Research on the Thymus (JART) proposed a CT-based division of the mediastinum into the following four compartments: superior mediastinum, anterior mediastinum (prevascular zone), middle mediastinum (peritracheoesophageal zone), and posterior mediastinum (paravertebral zone). JART did not include the cardiovascular system in their schematic.14 This model recognizes that thyroid goiters primarily remain confined to the superior mediastinum. Perhaps because they introduce complexity, there is a perception that four-compartment models are used less frequently than three-compartment models, by both radiologists and other clinicians.2,15
A practical CT-based mediastinal compartment scheme by the ITMIG is a clinical classification that divides the mediastinum into three compartments: (1) the prevascular (anterior), (2) visceral (middle), and (3) paravertebral (posterior) compartments, with clearly defined anatomic boundaries (Fig. 3.2).2,13 A disadvantage of the three-compartment model is the lack of division between the superior and anterior mediastinal compartments. However, it is advantageous because it is less complex, based on true anatomic planes, and bears similarity to widely known, published anatomic, clinical, and radiologic three-compartment models that are already in use.2,13 For standardization, the ITMIG definitions of the mediastinal compartments shall be utilized in this discussion.
Anterior (Prevascular) Mediastinal Compartment
The anterior or prevascular mediastinal compartment is bounded superiorly by the thoracic inlet, inferiorly by the diaphragm, anteriorly by the posterior cortex of the sternum, laterally by the mediastinal parietal pleural reflections,
and posteriorly by the anterior aspect of the pericardium. The major contents of this compartment include the thymus, lymph nodes, mediastinal fat, and left brachiocephalic vein.2,13,15
and posteriorly by the anterior aspect of the pericardium. The major contents of this compartment include the thymus, lymph nodes, mediastinal fat, and left brachiocephalic vein.2,13,15
Middle (Visceral) Mediastinal Compartment
The middle or visceral mediastinal compartment is defined superiorly by the thoracic inlet, inferiorly by the diaphragm, and anteriorly by the anterior aspect of the pericardium. This compartment is defined posteriorly by a vertical line located 1 cm posterior to the anterior margins of the thoracic vertebral bodies. This posterior margin is called the visceral-paravertebral compartment boundary line. The major contents of this compartment include both cardiovascular structures (heart, superior vena cava [SVC], ascending thoracic aorta, aortic arch, descending thoracic aorta, intrapericardial pulmonary arteries, and thoracic duct) and nonvascular structures (trachea, carina, esophagus, and lymph nodes). In the ITMIG classification, the extrapericardial pulmonary arteries and veins are considered to be pulmonary and not mediastinal structures.2,13,15
Posterior (Paravertebral) Mediastinal Compartment
The posterior or paravertebral mediastinal compartment is defined superiorly by the thoracic inlet, inferiorly by the diaphragm, anteriorly by the visceral compartment, and posterolaterally by paired vertical lines along the posterior margin of the chest wall at the lateral aspects of the transverse processes. The major contents of the paravertebral compartment include the thoracic spine and paravertebral soft tissues.2,13,15
Anatomic Variants
Normal Thymus
At birth, the normal thymus is an encapsulated, bilobed, H-shaped structure in the anterior mediastinum that has already reached its greatest weight (˜13 to 15g) relative to the rest of the body. By puberty, it has grown to its greatest absolute weight of 25 to 45 g.5,6,16 After puberty, it gradually involutes until only a remnant replaced by a fibrofatty tissue persists.5,6,16,17 The upper portion of the thymus typically lies anterior to the left innominate vein as it joins the right innominate vein to form the SVC, the proximal ascending
aorta, and the pulmonary outflow tract. It is sensitive to any kind of bodily stress, including systemic infection, neoplasm, surgery, and chemotherapy. It responds to these stressors with rapid atrophy, but regrows to its original size or even becomes larger after recovery.16,17
aorta, and the pulmonary outflow tract. It is sensitive to any kind of bodily stress, including systemic infection, neoplasm, surgery, and chemotherapy. It responds to these stressors with rapid atrophy, but regrows to its original size or even becomes larger after recovery.16,17
On frontal chest radiography, the normal thymus is often seen as a smoothly marginated soft tissue density causing mediastinal widening. It is usually difficult to distinguish from the cardiac silhouette and may even be mistaken for a mass in young children. The anterior reflections of the ribs produce a wavy contour on the soft, pliable thymus known as the “thymic wave” sign (Fig. 3.3), which is a helpful clue for identifying the normal thymic shadow.5,6,17 Normal thymus does not usually cause mass effect on the airway or adjacent vascular structures, in contradistinction to pathologic masses that commonly have irregular or lobular margins and cause mass effect. In 5% of children, a triangular extension of the normal right thymic lobe is seen, with a convex lateral border and a straight inferior border demarcated by the minor fissure. This creates a sail-like shadow, referred to as the “thymic sail” sign (Fig. 3.4).5,6,16,17 The thymic shadow becomes less evident around 2 years of age and is typically not evident on radiographs of children over 8 years of age.6
On ultrasound (US), the thymus of the neonate and young child has a distinctive heterogeneous echotexture but is hypoechoic relative to the liver and spleen. It has punctate and linear echogenic foci representing interspersed fat, giving it a “starry sky” appearance (Fig. 3.5A). With age, the thymus becomes more homogeneous and echogenic due to increasing fat content. On real-time US, the shape of the thymus varies with cardiac and respiratory motion, which is helpful in differentiating normal thymic tissue and anatomic variants from pathologic masses.5,6,16,17
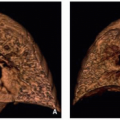
On enhanced CT, the thymus is a quadrilateral shaped soft tissue density structure with convex margins within the anterior mediastinum. With increasing age, the thymus becomes triangular in shape, with straight or concave margins. It has a homogeneous appearance and is hyperdense compared to the cardiac and chest wall musculature in infants, and nearly isodense later in childhood (mean Hounsfield Unit values range from 80.8 in infancy to 56.4 at 14 years of age) (Fig. 3.5B). Attenuation of the thymus declines with increasing age due to fatty replacement and cellular involution.5,6
On MRI, the thymus shows homogeneous signal higher than that of muscle on T1-weighted images and intermediate signal intensity on T2-weighted images (Fig. 3.5C). Reflecting intracellular fat content, thymic tissue has homogeneously decreased signal intensity on opposed-phase MRI relative to that seen on in-phase imaging. Chemical shift imaging has been used to assess the degree of fatty replacement and identify infiltrative disorders.5,16,18 MRI is able to represent thymic size more accurately than CT because of better contrast resolution. 5,18 The thickness of the left lobe of the thymus can be used as an indicator of thymic enlargement. In patients <20 years of age, the normal maximum dimension is 18 mm, measured perpendicular to the axis of the aortic arch.19
Normal Thymic Variants
Cross-sectional imaging has been helpful in identifying normal thymic anatomical variants in unusual locations in the mediastinum and neck. A rare congenital variant is the retrocaval thymus that extends posterior to the SVC (Fig. 3.6). This variant can displace the SVC laterally and mimic a mediastinal mass or right upper lobe collapse on chest radiographs. Cervical or suprasternal thymic extension to the level of thyroid gland is a frequently encountered variant, seen in up to 67% of infants and young children (Fig. 3.7). Such extension is more externally visible with increased intrathoracic pressure and can sometimes alarm parents or guardians.6 Normal superior extension of the thymus must be distinguished from accessory or ectopic cervical thymic tissue, which can be found anywhere along the path of embryologic thymic descent, from the angle of the mandible to the superior mediastinum. This uncommon cause of
pediatric neck mass is believed to be caused by arrest of normal descent or sequestration of a thymic rest during caudal migration. The accessory tissue can be solid, cystic, or a combination of both.6,20
pediatric neck mass is believed to be caused by arrest of normal descent or sequestration of a thymic rest during caudal migration. The accessory tissue can be solid, cystic, or a combination of both.6,20
Ectopic Parathyroid Gland
The parathyroid glands are yellowish-brown, flat, ovoid structures that measure ≈3 × 6 mm. They originate from the endoderm and develop from the dorsal wings of the third and fourth pharyngeal pouches. These are small endocrine glands that produce parathyroid hormone, which plays a role in calcium regulation in the blood and bones. There are typically two pairs of parathyroid glands (one superior and one inferior pair); each gland weighs 30 to 40 mg and is usually located at the upper, posterolateral surface of the thyroid gland. More than 4, and rarely less than 4, parathyroid glands have been described.
Ectopic parathyroid glands result from aberrant migration of the parathyroids during early development. Their prevalence ranges from 28% to 42.8% in autopsy series, although lower rates (2%) have been reported. The inferior parathyroids are more frequently ectopic than the superior glands and are most often found in close proximity to thymic tissue in the mediastinum.21,22 Although, the differential diagnosis depends on location, ectopic parathyroids must be differentiated from lymph nodes, thyroid, and thymic tissue. Like orthotopic parathyroid glands, ectopic parathyroids can develop adenomas and present with symptoms of hyperparathyroidism. The incidence of ectopic parathyroid adenoma is reported to be 6% to 25%.21
99mTc-sestamibi scintigraphy (MIBI) is often used for preoperative localization of parathyroid adenomas, with reported sensitivity of 71% to 93%.21 Ectopic parathyroids may be detected with MIBI with almost the same sensitivity as orthotopic adenomas (Fig. 3.8). Other nuclear imaging studies such as single photon emission computed tomography (SPECT), alone or in combination with CT (SPECT/CT), improve parathyroid adenoma localization. Dual-energy CT (DECT), which acquires two data sets showing different attenuation values and may quantify iodine contrast uptake in soft tissues, has successfully detected ectopic parathyroid adenomas in cases where routine methods have not.22 US is commonly used to locate hyperplastic parathyroid glands and adenomas, but it depends on the skill and expertise of the operator. Sensitivity varies from 44% to 87% and is lower (≤30%) for locating ectopic parathyroid glands.21,22 The use of CT for detection of enlarged parathyroid glands in patients who have not had previous exploratory surgery has shown sensitivity
values ranging from 76% to 83%, but sensitivity is even higher (up to 100%) when helical CT is combined with MIBI.21 Preoperative MRI is useful when CT is not performed.23
values ranging from 76% to 83%, but sensitivity is even higher (up to 100%) when helical CT is combined with MIBI.21 Preoperative MRI is useful when CT is not performed.23
Mediastinal parathyroid adenomas are usually resected via cervical incision. However, in 2% of cases, the ectopic gland is not accessible via this approach and video-assisted thoracoscopy combined with intraoperative MIBI may be used to minimize the extent of operative dissection.22,23 Robotic thoracoscopic approaches have also been developed for mediastinal parathyroids.22
IMAGING TECHNIQUES
A thorough history and physical examination focused on signs and symptoms that guide decisions on diagnostic testing remains the most important step in mediastinal mass
evaluation. Forty-eight to sixty-two percent of patients with mediastinal masses are symptomatic at the time of diagnosis, with anterior mediastinal lesions more commonly causing symptoms than middle or posterior mediastinal lesions.4
evaluation. Forty-eight to sixty-two percent of patients with mediastinal masses are symptomatic at the time of diagnosis, with anterior mediastinal lesions more commonly causing symptoms than middle or posterior mediastinal lesions.4
The major goals of imaging mediastinal masses include (1) identification and accurate compartmental localization, (2) detailed mass description and characterization, (3) provision of an appropriate differential diagnosis, and (4) recommendation of a cost-effective imaging and patient management plan.1,15 The various imaging modalities currently available for mediastinal mass evaluation are chest radiography, US, CT, MRI, and nuclear medicine studies.
Since most mediastinal lesions occur in a particular location with consistent frequency, lesion localization aids in the formulation of a differential diagnosis, helps in initiating treatment plans and strategies, and facilitates communication among clinicians in multiple disciplines. It is not always easy to determine the origin of large masses, which may involve more than one mediastinal compartment. In some cases, large mediastinal masses can mimic lung or even chest wall pathology. There are two methods aimed at improving the process of compartment localization. The first is the “center method,” which analyzes the lesion in multiple planes to identify the location of its center. The center of the lesion theoretically represents the site of origin, and this method has been shown to be capable of accurately assigning masses to specific mediastinal compartments. The second method is the “structure displacement tool,” which evaluates the degree and direction of effacement and shifting of mediastinal structures in order to localize the compartment of origin.2,15
After localization and compartmentalization, careful characterization of the mass should be performed. Masses can be solid, cystic, or of mixed composition. It is also important to detect calcifications, fat, and hemorrhagic components, as well as evaluate the pattern of contrast enhancement. The extent of the mass and invasion of adjacent organs must also be carefully considered. With this information, an accurate differential diagnosis can be proposed, and in some cases, a specific diagnosis can be given.
Radiography
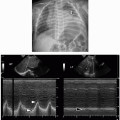
Imaging investigation for mediastinal masses usually begins with chest radiography. It is low cost, readily available, and well tolerated by children, and no specific preparation is needed. It is important to obtain both frontal and lateral chest radiographs in as many cases as possible. In infants and very sick pediatric patients, a cross-table lateral radiograph can be obtained in lieu of an upright lateral view. Although small mediastinal lesions may not be readily apparent on radiographs, large masses may manifest in various ways, including with loss of normal mediastinal contours or “silhouette” sign, defined as obscuration of normal tissue interfaces (Fig. 3.9).13 Chest radiographs have much lower sensitivity and specificity for mediastinal masses than cross-sectional imaging techniques, and additional imaging tests should be considered in cases of ongoing clinical suspicion and negative or equivocal screening radiography.1
Ultrasound
Ultrasound (US) is portable, useful in real time, widely available and does not utilize ionizing radiation. These factors make it especially useful in the pediatric population. In infants and younger children, US can be used to differentiate normal thymus from pathologic mediastinal masses. However,
it has a limited role in the evaluation of mediastinal masses in adults and children over 5 years of age due to suboptimal acoustic windows (due to ossification of the sternum). As in other applications, it is also important to consider that US is operator dependent.1,15,24
it has a limited role in the evaluation of mediastinal masses in adults and children over 5 years of age due to suboptimal acoustic windows (due to ossification of the sternum). As in other applications, it is also important to consider that US is operator dependent.1,15,24
Transducer selection is based on patient age and size. A 5- to 10-MHz linear-array transducer is used in infants and neonates, while a 2- to 4- or 4- to 7-MHz linear-array or sector transducer is used in older children and adults. With the patient lying supine, imaging can be obtained utilizing a suprasternal, parasternal, sternal, subxiphoid, or intercostal approach. Prone and decubitus positioning may also be needed, depending on the mediastinal compartment(s) involved. Doppler imaging is useful for assessing lesional vascularity and relationships with the blood vessels and heart.15
Computed Tomography
Computed tomography (CT), specifically multidetector CT (MDCT), plays an important role in evaluating mediastinal abnormalities despite the risks associated with ionizing radiation and iodinated contrast. In the last 15 to 20 years, MDCT has become more widely available, with faster imaging acquisition that has significantly decreased the need for sedation in infants and young children. It has excellent temporal and spatial resolution, as well as multiplanar and 3D reconstruction capabilities that allow for detailed evaluation of mediastinal structures. Compared to radiography and US, CT is overall superior, with greater accuracy in detecting, characterizing, and compartmentalizing mediastinal masses.1,15,25 Specific imaging findings that should be noted on CT evaluation of mediastinal lesions include (1) location, size, and configuration; (2) attenuation, heterogeneity, and enhancement; (3) presence of intralesional fat, cystic components, and soft- tissue calcification; and (4) connection to or invasion of adjacent structures (Fig. 3.10).13
Recently, utilization of CT perfusion has allowed rapid, quantitative assessment of tissue hemodynamics, which is sometimes helpful in tumor staging and evaluation of therapeutic response. CT perfusion can also help differentiate thymoma from other malignant lesions of the anterior mediastinum, including lymphoma, thymic carcinoma, and invasive lung cancer.26 Radiation-related risks remain an important concern in pediatric patients, particularly those who need multiple CT studies. Thus, a clear indication for the study is a requirement, and appropriate technical parameters and application of radiation dose reduction techniques are important. Low kilovolt potential (kVp) imaging, automatic tube current modulation to minimize milliamperage (mA), and image reconstruction techniques such as iterative reconstruction can be used to lower the radiation dose.27,28
MDCT facilitates the assessment of extracardiac systemic and pulmonary arterial and venous structures. It is advantageous due to rapid acquisition time, which lessens the need for sedation or general anesthesia. MDCT yields images with better temporal and spatial resolution, greater anatomic coverage, more reproducible assessment of lesional enhancement, and higher quality 2D reformation and 3D reconstruction capabilities, which are all important in understanding complex mediastinal vascular anomalies, planning surgeries, and evaluating posttreatment changes.28,29
In children, CT angiography (CTA) requires administration of low- or iso-osmolar intravascular iodinated contrast agents (300 mg I/mL or greater). The volume of contrast material injected is usually weight-based, ranging from 1.0 to 3.0 mL/kg.28,29,30 Some authors advocate contrast and normal saline dilution with a 1:1 ratio in order to minimize streak artifact.28 A power injector is generally preferred over manual injection to obtain homogeneous vascular opacification, but manual injection is used in instances with challenging venous access where only smaller IV catheters can be placed.29,30 Recent studies have shown that manual injection can produce diagnostic-quality thoracic aortic and central pulmonary arterial CTA in pediatric patients with small gauge IV catheters.31,32 For evaluation of extracardiac congenital mediastinal vascular anomalies in children, electrocardiographic gating is usually not needed.28,30
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) has been increasingly utilized for mediastinal mass evaluation in children and adults due to its superior tissue-contrast resolution and lack of associated ionizing radiation dose. MRI provides both physiologic and anatomic information. MRI protocols vary among institutions, depending on available equipment and preferences of the interpreting radiologists. Mediastinal lesions are generally imaged using multipurpose body/torso coils, but cardiac coils can be used for smaller infants. Some posterior lesions are most optimally imaged with spinal coils. Cardiac and respiratory motion compensation/correction techniques are sometimes necessary to produce a diagnostic MRI study.33
T1- and T2-weighted sequences in multiple planes are standard. Fat-suppressed and gadolinium-enhanced sequences are also important for tissue characterization and assessment of vascularity of mediastinal structures and masses (Fig. 3.11A). Chemical shift MRI has been shown to be useful in distinguishing normal thymus from thymic neoplasms and lymphoma. Diffusion-weighted MRI (DWI) is another advanced sequence that shows biophysical differences between tissues. Studies have shown that the mean apparent diffusion coefficient value of malignant mediastinal disease is significantly lower than that of benign disease.13,25
MRI has shown special utility in evaluating mediastinal masses with respect to (1) problem solving in cases of foregut duplication cyst with high attenuation on noncontrast CT and (2) evaluation of neurogenic tumors in the posterior mediastinum, including assessment for intraspinal extension.1 MRI has also proven useful in evaluating postoperative fluid collections or abscesses that have complicated mediastinal surgeries.33
MR angiography (MRA) with intravenous gadolinium contrast can be used as an alternative to conventional angiography and is capable of imaging the circulatory system in a noninvasive fashion. Unlike conventional angiography, acquired images not only evaluate the vascular lumen but also the vessel wall and surrounding mediastinal structures. This technique increases the accuracy for detection of extracardiac mediastinal vascular abnormalities, both congenital and acquired (Fig. 3.11B).34,35 Advantages of MRA include a wide field of view, multiplanar imaging capability, and adequate spatial resolution for detection of vascular rings and associated airway anomalies, without the use of ionizing radiation or iodinated contrast material. Disadvantages of MRA include limited availability, higher cost, long acquisition times, and the need for deep sedation or general anesthesia in young children.27,28,29
Nuclear Medicine
Positron emission tomography (PET) utilizing fluorodeoxyglucose (18F-FDG) injection coregistered with CT (PET/CT) is a nuclear medicine study that is useful for evaluating a subset of mediastinal masses. Although PET/CT is not currently the first-line modality, it has become important in tumor staging and assessment of treatment response. Because this test is a fusion of two modalities, it provides both functional and
anatomic information. PET has proven to be especially useful in lymphoma evaluation when other imaging modalities have limitations. Its role includes (1) detection of tumor in normal-sized lymph nodes, (2) differentiation between scar or nonviable residual tissue and viable active tumor following treatment, and (3) evaluation of disease in extranodal sites.1
anatomic information. PET has proven to be especially useful in lymphoma evaluation when other imaging modalities have limitations. Its role includes (1) detection of tumor in normal-sized lymph nodes, (2) differentiation between scar or nonviable residual tissue and viable active tumor following treatment, and (3) evaluation of disease in extranodal sites.1
Metaiodobenzylguanidine (MIBG) scan is a scintigraphic study that utilizes MIBG labeled with 123I or 131I as a radiotracer. MIBG is a guanethidine analog, similar to norepinephrine. Chromaffin cells within abnormal sympathetic adrenergic tissue demonstrate elevated uptake of MIBG. MIBG scan is particularly helpful in evaluation of neuroblastoma in the posterior mediastinum, with a detection rate of 90% to 95%.15
SPECTRUM OF MEDIASTINAL
DISORDERS
Nonvascular Lesions
Anterior Mediastinal Lesions The most common masses in the anterior or prevascular mediastinal compartment are prominent normal thymus and abnormal thymic tissue affected by benign conditions such as thymic hyperplasia and neoplasms such as thymoma, thymic carcinoma, and neuroendocrine tumor. Other masses in this compartment are lymphoma, germ cell neoplasms, metastatic lymphadenopathy, and infectious processes. Prevascular compartment lesions usually displace the mediastinal vessels posteriorly and inferiorly.1
Soft Tissue Lesions
Thymic Hyperplasia
Thymic hyperplasia can be divided into two distinct types depending on its histological makeup: true hyperplasia and lymphoid (follicular) hyperplasia. True thymic hyperplasia is characterized by an increase in size and weight of the thymus with preservation of normal microscopic features. Although it may retain its normal bilobed appearance, most hyperplastic thymic glands become more oval in shape as they increase in size. Clinically, true thymic hyperplasia can be seen in three subgroups: patients without underlying medical conditions (Fig. 3.12A); those with disorders associated with thymic hyperplasia including hyperthyroidism, sarcoidosis, and red blood cell aplasia (Fig. 3.12B); and those with “rebound phenomenon” after recovery from a stressful medical condition such as pneumonia, corticosteroid therapy, radiation or chemotherapy, surgery, or burns.6,17,18 These physiologic stressors can lead to considerable decrease in thymic size, but the thymus usually returns to its original size after recovery, within ˜9 months.
In some pediatric patients with rebound hyperplasia, the thymus can grow to as much as 50% larger than its original size. This phenomenon is seen in 10% to 25% of patients who undergo chemotherapy, usually within 2 years of initiation of treatment (Fig. 3.13).17,18 Distinguishing thymic rebound hyperplasia from recurrent or metastatic neoplasm is sometimes a diagnostic dilemma. Rebound hyperplasia tends to show more diffuse enlargement, with maintenance of normal smooth contours and vascularity, with a fine mixture of fat and lymphoid tissue. On the other hand, neoplasms tend to be more nodular and heterogeneous, and may show areas of necrosis and calcification.17 On PET, a standardized uptake value (SUV) of <3.4 has been shown to be more suggestive of thymic rebound than neoplasia. However, this finding should be correlated clinically and considered in conjunction with other imaging features.6,36,37
Thymic lymphoid (follicular) hyperplasia, also known as autoimmune thymitis, is a distinct entity that is more commonly encountered in clinical practice. It is associated with autoimmune and endocrine diseases such as myasthenia gravis, thyrotoxicosis, Addison disease, systemic lupus erythematosus, connective tissue disorders, and even early stages of human immunodeficiency virus infection. It is characterized by inflammation and proliferation of lymphoid follicles and hyperplastic lymphoid germinal centers in the medulla of the gland, with increased number of lymphocytes and epithelial cells. On imaging, the thymus may appear normal in size,
which makes detection difficult. However, there may be diffuse thymic enlargement or a focal thymic mass.6,17,18
which makes detection difficult. However, there may be diffuse thymic enlargement or a focal thymic mass.6,17,18
Thymoma
Thymomas are benign or low-grade malignant tumors arising from the medullary thymic epithelium that are characterized by the presence of a variable number of immature, nonneoplastic T cells. Thymomas are the most common tumors of the thymus and the most common anterior mediastinal neoplasms in adults (peaking in the 50 to 60 age group); however, thymomas account for <5% of mediastinal tumors in children.37,38 They affect males and females equally. Patients with thymoma are frequently asymptomatic,37 but 20% to 30% present with cough, chest pain, dyspnea, dysphagia, hoarseness, or SVC syndrome, which are symptoms related to local invasion and mass effect on adjacent structures. One-third to one-half of patients with thymoma develop myasthenia gravis, but only 15% of patients with myasthenia gravis are found to have a thymoma. The association between myasthenia gravis and thymoma is even more rare in children.17,18,38 Thymomas may also develop in patients with pure red blood cell aplasia, hypogammaglobulinemia, connective tissue disease, autoimmune disease, or inflammatory bowel disease. Almost 20% of patients with thymomas have concomitant malignancies such as lymphoma or lung or thyroid carcinoma.17,18
On radiographs, thymomas appear as sharply marginated, retrosternal mediastinal opacities with smooth or lobulated contours that can obscure the heart border. On CT, they are well-defined round, oval, or lobulated masses with homogeneous soft tissue attenuation, characteristically draping over the heart. There is generally homogeneous enhancement, but areas of cystic or necrotic degeneration may be present in around 30% (Fig. 3.14),15,17,18 and calcification may be present in the capsule or interspersed throughout the mass. Thymomas usually lead to loss of normal thymic shape. They can be subdivided into invasive and noninvasive forms, but this can only be definitively determined on a histopathologic basis, by demonstration of tumor beyond the confines of the fibrous capsule.15 Imaging signs suggestive of invasion include encasement of other mediastinal structures, infiltration of fat planes, and an
irregular interface between the mass and lung parenchyma. Pleural thickening, nodularity, or effusion also generally indicates pleural invasion. On MRI, thymomas appear as homogeneous or heterogeneous masses (sometimes with cysts, necrosis, and hemorrhage) with low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences (Fig. 3.15). Chemical shift artifact can be helpful in differentiating thymic hyperplasia from thymomas and other thymic tumors.6,17,18,37 FDG avidity may be seen on PET but is not reliable in discriminating among normal thymus, thymic hyperplasia, and thymoma. PET imaging can be helpful for detecting transdiaphragmatic spread and distant metastasis.18
irregular interface between the mass and lung parenchyma. Pleural thickening, nodularity, or effusion also generally indicates pleural invasion. On MRI, thymomas appear as homogeneous or heterogeneous masses (sometimes with cysts, necrosis, and hemorrhage) with low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences (Fig. 3.15). Chemical shift artifact can be helpful in differentiating thymic hyperplasia from thymomas and other thymic tumors.6,17,18,37 FDG avidity may be seen on PET but is not reliable in discriminating among normal thymus, thymic hyperplasia, and thymoma. PET imaging can be helpful for detecting transdiaphragmatic spread and distant metastasis.18
Thymomas are best treated by total surgical excision. Radiation therapy and chemotherapy may be required for advanced stage or partially resected or unresectable disease.38
Thymic Carcinoma
Thymic carcinomas account for about 20% of thymic epithelial tumors.25 These are uncommon, histologically malignant mediastinal neoplasms in adults that are even more rare in children. Thymic carcinomas behave aggressively and are likely to present with mediastinal nodal and distant metastasis (50% to 65%) at the time of diagnosis.17,18 Thymic carcinomas usually lack a well-defined capsule, whereas thymomas are encapsulated in up to two-thirds of cases. This group of tumors freely invades and commonly compresses adjacent mediastinal structures, leading to symptoms. Thymic carcinomas rarely cause paraneoplastic syndromes such as myasthenia gravis.17,18
On CT, thymic carcinomas usually appear as large, multilobulated, heterogeneous masses that may contain areas of necrosis and calcification. Their appearance on imaging is difficult to distinguish from that of thymomas. Features such as local invasion, distant metastasis, and associated mediastinal lymphadenopathy favor thymic carcinoma (Fig. 3.16). On MRI, thymic carcinoma shows high signal intensity on both T1- and T2-weighted sequences, with areas of heterogeneity due to necrosis or hemorrhage. Because this tumor is uncommon, particularly in children, other differential considerations should also be raised.17,18 It has been previously reported that high SUV on FDG-PET imaging may help to differentiate thymic carcinoma from thymoma, thymic hyperplasia, and normal thymus.17,18
Lymphoma
Lymphoma is the most common anterior mediastinal mass in children.33,39 Lymphoma has traditionally been divided into Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL). Lymphoma-related masses can rapidly grow and produce progressive airway compromise. Many affected children also present with life-threatening SVC syndrome and cardiovascular compromise, which is worsened in the supine position.33 HL is characterized histologically by the presence of multinucleated Reed-Sternberg cells, and NHL by T-lymphocyte or B-lymphocyte clonal proliferation.15 HL more commonly affects the thorax, with up to 85% having an anterior mediastinal mass at the time of diagnosis, and NHL has thoracic involvement in ˜50% of cases.6 HL typically occurs in the first decade of life, while NHL is common in both the first and second decades.1
Hodgkin Lymphoma. HL is divided into four main types: nodular sclerosing, mixed cellularity, lymphocyte depleted, and lymphocyte predominant. Nodular sclerosing is the most common subtype to present as a mediastinal mass.25,37 HL is staged using the Ann Arbor staging classification, based on the number of sites of lymph node involvement, the presence of extranodal disease, and the presence of clinical “B symptoms” such as fever, night sweats, and weight loss. The 5-year survival rate exceeds 90%.6,39 HL may manifest with isolated thymic involvement, isolated nodal involvement, or a combination of both. Differentiating primary thymic lymphomas from thymomas on the basis of imaging can be difficult, though thymic lymphomas typically occur in younger patients.
Non-Hodgkin Lymphoma. Non-Hodgkin lymphoma (NHL) accounts for ˜10% to 15% of all childhood cancers. There are four main types of NHL: lymphoblastic or T-cell, Burkitt, non-Burkitt, and large cell or histiocytic type. T-cell NHL commonly presents as a mediastinal mass.37 NHL is more frequent in patients under the age of ten, and males represent up to 70% of the affected patient population. NHL is staged according to disease extent and prognosis utilizing the St. Jude staging system, which divides disease into “limited” (stages 1 and 2) and “extensive” (stages 3 and 4) categories. The associated rapidly growing mediastinal tumors are accompanied by symptoms of dyspnea, cough, chest pain, and hoarseness, which all occur due to tumor mass effect. Affected pediatric patients can also present with SVC compression and symptoms of venous or lymphatic obstruction. Extrathoracic and extranodal manifestations are frequently seen with NHL.6
Lymphoma Imaging. On chest radiography, lymphoma may present as an incidental finding or as nonspecific prevascular mediastinal widening.39 CT or MRI is often obtained for further evaluation, demonstrating discrete or confluent lymphadenopathy, or a large, homogeneous, lobulated, mildly to moderately enhancing mass with soft tissue attenuation on CT or signal intensity on MRI (Fig. 3.17).15,25 The mass is typically midline in location, displacing adjacent normal structures and sometimes involving the thymus. Pleural effusions, pulmonary nodules, and chest wall involvement are seen in some cases. Calcifications are rare prior to treatment.15,39 With increasing size, lymphomatous masses may develop central areas of necrosis and hemorrhage.6,25 Lymphoma resolves after appropriate therapy in the majority of patients, with residual soft tissue density that generally represents fibrosis.17
Advanced MRI techniques are useful in the imaging of lymphoma. DWI has shown great promise in tumor detection and assessment of treatment response in lymphoma patients. In addition, dynamic contrast-enhanced MRI has been shown to be useful in differentiating lymphoma and thymoma, with lymphoma demonstrating a longer time to peak enhancement.15
PET/CT has been utilized in lymphoma imaging, with the advantages of being able to detect disease in nonenlarged lymph nodes, bone marrow, and other extranodal sites, and help guide biopsy to sites of the most metabolically active
tissue. PET/CT has also been able to suggest the aggressiveness of the tumor based on the degree of FDG avidity (Fig. 3.18).37,40
tissue. PET/CT has also been able to suggest the aggressiveness of the tumor based on the degree of FDG avidity (Fig. 3.18).37,40
Until recently, routine surveillance of treated lymphoma entailed annual or even more frequent CT examinations, typically for up to 5 years after therapy. Some protocols also included PET/CT for routine surveillance. Recently, it has been found that the only predictor of overall survival is the time to relapse: patients relapsing within the first year after completing therapy have lower overall survival than do those with later relapses. This suggests that routine surveillance imaging after 1 year is unlikely to impact overall survival.6,41
Fat-Containing Lesions
Germ Cell Tumors
Germ cell tumors (GCT) comprise a heterogeneous group of benign and malignant neoplasms originating from primitive germ cells. They usually develop in the midline, anywhere from the pineal region to the sacrococcygeal region. The anterior mediastinum is the most common site of extragonadal germ cell tumors. More than 80% of these are benign. GCTs account for 1% to 15% of mediastinal tumors in adults and about 6% to 25% of mediastinal tumors in children.1,15,17,37,38 Specific tumor types include benign (mature) and malignant (immature) teratoma and nonteratomatous GCTs. While benign tumors have no gender predilection, ˜90% of malignant germ cell tumors are seen in males.
Teratoma. Teratomas are the most common form of mediastinal GCT, representing 80%.18,38 Benign, mature mediastinal teratomas are well-differentiated tumors that are curable with surgery. Benign teratomas are commonly asymptomatic and are discovered as anterior mediastinal masses on routine chest radiographs in older children, but symptoms from compression or erosion of adjacent structures may occur.17,18,38
On imaging, teratomas commonly appear as well-defined rounded or lobulated masses, which are often large, protruding off midline at the time of diagnosis. The mass can have solid and cystic areas, presenting a multilocular cystic appearance. Fatty components are commonly seen (90%) (Fig. 3.19).17,18,38 Since teratomas contain derivatives of all three germ cell layers, they may contain teeth, bone, or calcification (seen in 20% of cases) in addition to hair, sebaceous glands, and muscle.17,18,38 The combination of soft tissue, cystic areas, fat, and calcification on imaging is highly suggestive of benign, mature teratoma, although malignant tumors may have the same appearance. A fat-fluid level within the mass is diagnostic of teratoma but is seen in only about 10% of cases.17,18 Malignant (immature) teratomas represent 14% of teratomas and may have more nodular soft tissue components, with areas of necrosis or hemorrhage. Only 40% of malignant teratomas contain fat, and they exhibit poorly defined margins that show thick enhancement following contrast administration. Pleural effusion may be present.15,17,18
Surgical resection is indicated to relieve symptoms and exclude malignant elements. Follow-up imaging is required, because any recurrence has a high risk of harboring malignancy.38
Nonteratomatous Germ Cell Tumors. Nonteratomatous GCTs are rare malignant tumors of the mediastinum, occurring predominantly in young adults.42 They are generally divided into two broad groups: seminomatous and nonseminomatous GCT.15,42,43 Seminomas are malignant neoplasms
containing a single histological cell type that tend to occur in Caucasian men in the third and fourth decades of life. On imaging, seminomas are bulky, lobulated, homogeneous masses that rarely calcify (Fig. 3.20). Although local invasion is infrequent, metastases to regional lymph nodes and bone may be seen in advanced stages.15,18 Seminomas are extremely chemo- and radiosensitive.42
containing a single histological cell type that tend to occur in Caucasian men in the third and fourth decades of life. On imaging, seminomas are bulky, lobulated, homogeneous masses that rarely calcify (Fig. 3.20). Although local invasion is infrequent, metastases to regional lymph nodes and bone may be seen in advanced stages.15,18 Seminomas are extremely chemo- and radiosensitive.42
Nonseminomatous GCTs include endodermal sinus tumor (yolk sac tumor), embryonal carcinoma, teratocarcinoma, choriocarcinoma, and mixed tumors with both differentiated and undifferentiated elements.42,43 These tumors rarely occur in the mediastinum (2% to 5% of cases), but if they do, most are symptomatic, presenting with dyspnea, cough, chest pain, and hemoptysis due to compression of adjacent tissues.42,43 Associated conditions, such as hematologic malignancies (leukemia or myelodysplastic syndrome) and Klinefelter syndrome (47, XXY), have been reported.18,42
On cross-sectional imaging, these tumors are often large, irregular, and heterogeneous, with foci of necrosis and hemorrhage. Invasion of adjacent mediastinal structures is common, along with distant and regional node metastasis in advanced disease (Fig. 3.21). Pleural and pericardial effusions are common.15,18 Endodermal sinus tumor tends to recur locally and also has a high incidence of metastatic disease at the time of presentation.44 Teratocarcinomas are typically more nodular or poorly defined than benign teratomas, demonstrate less fatty component, appear more solid, and show a thick enhancing capsule after administration of contrast.45 Seminoma and nonseminomatous GCTs may be difficult to distinguish from other malignant anterior mediastinal masses by imaging (CT and/or MRI) alone. Correlation with other clinical and laboratory parameters including elevated tumor markers, such as alpha-fetoprotein and/or human chorionic gonadotropin, may yield a diagnosis.15
Lipoma
Mediastinal lipoma is a rare condition in pediatric patients. Lipomas are well-encapsulated fatty masses with an identical composition to subcutaneous fat. They occur predominantly
in the anterior mediastinum, representing 1.6% to 2.3% of all primary mediastinal tumors.46 Due to their slow growth and pliability, affected pediatric patients are usually asymptomatic, and the mass is incidentally detected on imaging performed for a different reason.15,47 While these tumors are benign, they can increase in size and cause symptoms, and rare recurrences have been reported following resection.47
in the anterior mediastinum, representing 1.6% to 2.3% of all primary mediastinal tumors.46 Due to their slow growth and pliability, affected pediatric patients are usually asymptomatic, and the mass is incidentally detected on imaging performed for a different reason.15,47 While these tumors are benign, they can increase in size and cause symptoms, and rare recurrences have been reported following resection.47
On chest radiography, lipomas are well-defined masses with convex borders that may be less dense than the adjacent soft tissues. CT shows a homogeneous fatty composition (approximately -100 HU) with smooth, well-demarcated borders (Fig. 3.22). Lipomas are hyperintense on both T1-weighted and T2-weighted sequences, and signal suppresses with fat-suppression techniques. There is no internal enhancement.15,46
Liposarcoma
Liposarcoma is a rare, malignant soft tissue tumor derived from primitive embryonic tissues. Although mediastinal liposarcoma is primarily an adult disease, it may also occur in children and young adults.48 Commonly occurring in the prevascular compartment, it is the most common malignant mediastinal mesenchymal tumor. Specific histological types have potential for metastasis. Well-differentiated liposarcomas are the least aggressive, while dedifferentiated and myxoid liposarcomas can disseminate to the pleural, pericardial, and diaphragmatic surfaces. Pleomorphic sarcomas are rare, and mixed-type tumors also occur.48,49 Affected patients may be asymptomatic, with a mass found incidentally on imaging obtained for an alternative indication. If symptomatic, patients may present with tachypnea, SVC syndrome, chest pain, and weight loss.15
Typical imaging findings of mediastinal liposarcoma include mediastinal widening on chest radiographs and possible tracheal deviation. On cross-sectional imaging, it can have a variable appearance, ranging from a predominantly fatty mass to a large, heterogeneous solid mass lesion with little to virtually no visible macroscopic fat. Liposarcoma should be the primary consideration in cases with large, prevascular mediastinal masses containing predominantly enhancing soft tissue components with little interspersed fat (Fig. 3.23). It may be difficult to distinguish liposarcomas from lipomas, thymolipomas, and fatcontaining mediastinal germ cell tumors based on imaging findings alone.15
The treatment for mediastinal liposarcoma is surgical resection, often with adjuvant chemotherapy and radiation therapy.49 Although the overall prognosis is poor, it depends
upon the histologic subtype and completeness of surgical excision.48
upon the histologic subtype and completeness of surgical excision.48
Thymolipoma
Thymolipomas are rare, benign, well-encapsulated thymic tumors that account for about 2% to 9% of all thymic neoplasms.45 They can occur at any age (mean age, 21 years) and have no sex predilection.17,25 Pediatric patients with thymolipomas are usually asymptomatic but may present with compression-related symptoms. Thymolipomas are often large but malleable, with a tendency to conform to adjacent structures, extend to the cardiophrenic and costophrenic angles, or even occupy almost an entire hemithorax.17 In rare cases, thymolipomas are associated with myasthenia gravis, aplastic anemia, thyrotoxicosis, or Graves disease.45
On chest radiography, mediastinal widening or a large, well-marginated anterior and inferior mediastinal mass is seen. The mass is less dense than adjacent soft tissues due to inherent fat content. It may mimic cardiomegaly, excessive epicardial fat, diaphragmatic elevation, lobar collapse, or a pericardial cyst or effusion. On CT, thymolipomas are large and predominantly show fat (low) attenuation intermixed with fibrous septa and normal thymic tissue (Fig. 3.24). On MRI, thymolipomas show heterogeneous high signal intensity on T1- and T2-weighted sequences, along with strands of lower signal intensity representing fibrous septa.6,17 They may diffusely involve the thymus or appear as pedunculated masses arising from a normal-appearing thymus.18 When anatomy is clearly delineated, a connection to the superior mediastinum can often be identified.45
The current treatment of choice for thymolipoma is surgical excision.
Cystic Lesions
Thymic Cyst
Thymic cysts account for about 3% of anterior mediastinal masses and they can either be congenital or acquired. Congenital thymic cysts originate from embryonic remnants and may be found anywhere along the course of the embryonic thymopharyngeal duct, which extends from the upper neck to the anterior mediastinum.17 They are usually asymptomatic and discovered incidentally.18 Acquired thymic cysts have been reported to occur before and after chemotherapy for lymphoma, after thoracotomy, in patients with thymomas and other thymic tumors, and in patients with germ cell tumors. Multilocular thymic cysts have been reported in pediatric patients with human immunodeficiency virus infection.15,17,18
On radiographs, thymic cysts can be occult, but when large enough, they can be seen as homogeneous and smoothly marginated masses that may have rim calcifications. They are usually close to water in density, although this may vary depending on the presence of blood products or fat.6,17 On US, thymic cysts appear as anechoic masses that may contain proteinaceous debris if complicated by prior inflammation or hemorrhage. Septations can also be seen.6 On CT, congenital thymic cysts may show fluid attenuation or they may be higher in density, depending on their contents.
They have well-demarcated walls, sometimes with rim calcification and without central contrast enhancement (Fig. 3.25).6,18 Acquired thymic cysts tend to be multilocular, often with thicker walls, internal septations, and more gelatinous liquid contents.19 MRI typically shows low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences. However, if the cyst contains proteinaceous material, it may exhibit high signal intensity on both T1- and T2-weighted sequences.18,37
They have well-demarcated walls, sometimes with rim calcification and without central contrast enhancement (Fig. 3.25).6,18 Acquired thymic cysts tend to be multilocular, often with thicker walls, internal septations, and more gelatinous liquid contents.19 MRI typically shows low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences. However, if the cyst contains proteinaceous material, it may exhibit high signal intensity on both T1- and T2-weighted sequences.18,37
Lymphatic Malformation
Lymphatic malformations (LMs) are benign, lymph-containing multicystic lesions that result from congenital maldevelopment of the lymphatic channels. They may occur anywhere in the body, arising in the neck and axillary region in more than 80% of cases.25 Intrathoracic location is rare, seen in only 1% of cases.15,38 Approximately 50% are diagnosed at birth, and 90% are diagnosed by 5 years of age.33 As a rule, they do not respect fascial or spatial boundaries.15 Up to 15% are large enough to produce respiratory symptoms from airway compromise.33 Thoracic LMs are often incidental findings on chest imaging. As a result of compression of mediastinal structures and lung, affected pediatric patients may present with cough, stridor, dyspnea, dysphagia, hemoptysis, SVC syndrome, or Horner syndrome (ptosis, miosis, and anhidrosis).38 LMs are characterized as macrocystic (bearing cysts >1 cm in diameter), microcystic (cysts <1 cm in diameter), or combined.50
The size of an LM dictates its appearance on the chest radiograph. Small LMs may be occult, but large lesions tend to present with nonspecific, lobulated mediastinal widening that may extend to or emanate from the neck area. US can be utilized in young children but has limited ability to define the extent of the lesion. US typically demonstrates a heterogeneous, multicystic mass with no intralesional vascular flow on Doppler US imaging (Fig. 3.26).15 Echogenic components
correspond histologically to abnormal small lymphatic channels, hemorrhage, or even infected cystic spaces.51 CT demonstrates mostly multiseptated masses, which may insinuate around and compress normal mediastinal structures. The walls and septations may show mild enhancement (Fig. 3.27). MRI is commonly performed for lesion characterization, determination of extent, and assessment of the LM’s relationship to regional structures. Intracystic contents are most often hyperintense on T2-weighted sequences, with variable signal on T1-weighted sequences that is dependent on whether proteinaceous contents or blood products are present. Variable internal enhancement may be seen if there has been previous hemorrhage.15 Many patients with intrathoracic LMs can benefit from MRI mapping of the extent of the lesion in order to facilitate appropriate intervention.33
correspond histologically to abnormal small lymphatic channels, hemorrhage, or even infected cystic spaces.51 CT demonstrates mostly multiseptated masses, which may insinuate around and compress normal mediastinal structures. The walls and septations may show mild enhancement (Fig. 3.27). MRI is commonly performed for lesion characterization, determination of extent, and assessment of the LM’s relationship to regional structures. Intracystic contents are most often hyperintense on T2-weighted sequences, with variable signal on T1-weighted sequences that is dependent on whether proteinaceous contents or blood products are present. Variable internal enhancement may be seen if there has been previous hemorrhage.15 Many patients with intrathoracic LMs can benefit from MRI mapping of the extent of the lesion in order to facilitate appropriate intervention.33
Surgical excision of LMs was previously considered standard treatment and is still performed in some cases. Extensive lesions, however, tend to infiltrate muscle and distort nerves and vessels; therefore, complete surgical excision without compromise of vital structures is not always possible. Local injection of various sclerosing agents, including ethanol, ethibloc, sodium tetradecyl sulfate, bleomycin, and doxycycline, has also been utilized safely and yielded good results.16,38,50,51
Middle Mediastinal Lesions
Approximately 20% of mediastinal masses occur in the middle mediastinal compartment. The most common nonvascular middle mediastinal masses are developmental foregut malformations (bronchogenic, enteric, and neurenteric cysts). The middle mediastinal compartment is also a common location for lymphadenopathy secondary to infection, primary neoplasm, or metastatic disease.1,2,15 Lesions of the great vessels also occur in this compartment.
Congenital Lesions
Foregut Duplication Cysts
Foregut duplication cysts (FDCs) are congenital malformations resulting from abnormal embryonic foregut development. These are the most common primary middle mediastinal compartment masses, representing 11% of all pediatric mediastinal masses. FDCs are divided into three main histologic subtypes, namely bronchogenic, esophageal, and neurenteric cysts.15,52 Symptoms usually depend on the size and location of these cysts, which may cause compression of the trachea or bronchi leading to distal collapse and air trapping. Infection is also encountered in some cases.53
Bronchogenic Cyst. Bronchogenic cysts are the most common mediastinal cysts, resulting from abnormal lung budding during ventral foregut development.37,54 These fluid-filled lesions lined by respiratory epithelium are typically seen near the carina and in the paratracheal region.25,37,38,52 Although the middle mediastinal compartment is the most common location for bronchogenic cysts, they can also be seen in the other mediastinal compartments and lung parenchyma.37,38 These cysts are usually found in the first four
decades of life with approximately 42% occurring in the pediatric population. Because of their central location, large bronchogenic cysts tend to compress the large airways and can lead to respiratory distress.52,54 These lesions are usually stable in size, except when complicated by infection or hemorrhage. Approximately 40% of bronchogenic cysts are symptomatic, resulting in cough, dyspnea, or chest pain. Air within a bronchogenic cyst may be due to communication with the tracheobronchial tree or infection.25
decades of life with approximately 42% occurring in the pediatric population. Because of their central location, large bronchogenic cysts tend to compress the large airways and can lead to respiratory distress.52,54 These lesions are usually stable in size, except when complicated by infection or hemorrhage. Approximately 40% of bronchogenic cysts are symptomatic, resulting in cough, dyspnea, or chest pain. Air within a bronchogenic cyst may be due to communication with the tracheobronchial tree or infection.25
Esophageal Duplication Cyst. Esophageal duplication cysts, also known as enteric duplication cysts, result from maldevelopment of the posterior division of the embryonic foregut and are lined by gastrointestinal tract mucosa.52,55,56 Esophageal duplication cysts may be associated with other anomalies, including intestinal duplications, esophageal atresia and fistula, and spinal anomalies.38 They are uncommon lesions and are generally asymptomatic, with swallowing difficulty being the most common symptom.52,56,57 If the esophageal duplication cyst contains active gastric or pancreatic mucosa, hemorrhage and rupture of the cyst are possible complications. Most of these lesions are located adjacent to or within the esophageal wall and can lead to mural ulceration and bleeding.25,55,57
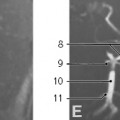
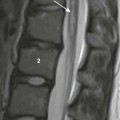
Neurenteric Cyst. Neurenteric cysts result from failure of separation of gastrointestinal tract from the primitive neural crest during early embryonic development. Histologically, both neural elements and gastrointestinal epithelium are typically seen lining these lesions. They are most commonly located in the posterior mediastinal compartment where they can extend into the spinal canal, but can also be seen in the middle mediastinal compartment. Associated congenital vertebral anomalies such as open dysraphism can be seen.15,25,37,52,57,58 Almost all neurenteric cysts are diagnosed by one year of age, usually presenting with symptoms of tracheobronchial compression, such as dyspnea, stridor, and persistent cough.57
Foregut Duplication Cyst Imaging. On chest radiographs, all three types of FDCs present as nonspecific, well-circumscribed round or ovoid soft tissue masses in the mediastinum. Small lesions may be obscured by other mediastinal structures and, therefore, be occult on radiographs. On CT, FDCs may have simple fluid attenuation, but mucus, hemorrhage, infection, and proteinaceous debris can increase attenuation (Fig. 3.28). This makes it difficult to differentiate FDCs from other mediastinal soft tissue masses on CT.15,37,56 No substantial internal enhancement is seen within FDCs, and there is, at most, minimal enhancement of their smooth walls.15,37,52
On MRI, FDCs are well-marginated cystic lesions with no or minimal cyst wall enhancement in uncomplicated cases. In 50% of cases, the cyst contents are uniformly hypointense on T1- and hyperintense on T2-weighted MRI, consistent with simple fluid (Fig. 3.29). If infection or intracystic hemorrhage has occurred, the fluid contents become more proteinaceous, with increased signal on T1-weighted sequences. A thick wall and mural calcification may help to distinguish esophageal duplication cysts from other FDCs.57 In cases complicated by infection, the cyst walls may become thickened and irregular and exhibit more robust enhancement. In these cases, MRI is particularly helpful, as fluid-fluid levels may be visible.15,37,52 It is difficult to differentiate bronchogenic cysts from esophageal duplication cysts on imaging,25 but neurenteric cysts are unique in their association with congenital vertebral anomalies (Fig. 3.30).15 99mTc-pertechnetate scan can help to identify esophageal duplication cysts containing ectopic gastric mucosa, which increases the risks of cyst rupture and hemorrhage.57
Infectious and Inflammatory Adenopathy
Tuberculosis
Tuberculosis (TB) is a transmittable disease caused by Mycobacterium tuberculosis. It is a global public health
problem and a leading cause of death and disability from infection. TB can affect every organ in the body, but pulmonary infection is by far the most common.59 Another important site of disease is regional lymph nodes. Younger children have a higher prevalence of lymphadenopathy, along with a variety of airway and other complications. Enlarged and edematous paratracheal, hilar, and subcarinal lymph nodes may encroach and compress upon the regional large airways, leading to significant respiratory symptoms.59
problem and a leading cause of death and disability from infection. TB can affect every organ in the body, but pulmonary infection is by far the most common.59 Another important site of disease is regional lymph nodes. Younger children have a higher prevalence of lymphadenopathy, along with a variety of airway and other complications. Enlarged and edematous paratracheal, hilar, and subcarinal lymph nodes may encroach and compress upon the regional large airways, leading to significant respiratory symptoms.59
On chest radiographs, lymphadenopathy usually presents as asymmetrically distributed, lobulated paratracheal, hilar, and/or subcarinal masses with sharp or ill-defined borders.
Enlarged lymph nodes can compress and displace the trachea and compress and stretch the bronchi. Lymphadenopathy is not always easy to detect on conventional radiographs, and CT has higher sensitivity. On enhanced CT, lymph nodes from TB infection may have a characteristic appearance, with central low attenuation and obliteration of perinodal fat. Lymph node calcifications may be seen in 15%.60 Prominent, irregular peripheral enhancement of the enlarged nodes, described as a “ghost-like” pattern of enhancement, has been described.61 Lymphadenopathy from TB can be significant and can coalesce to produce mass-like structures and eventually fibrosing mediastinitis, which is sometimes difficult to differentiate from other soft tissue masses (Fig. 3.31).15 US through a substernal window can be helpful in imaging TB lymphadenopathy, both for diagnosis and for follow-up after treatment in infants and young children. Abnormal lymph nodes are easily distinguished from blood vessels, thymus, and other mediastinal structures62,63 on US in these patients. MRI demonstrates TB lymphadenopathy in the mediastinum and may show low signal in the lymph nodes on T2-weighted MR images, with peripheral enhancement on T1-weighted postcontrast images.33
Enlarged lymph nodes can compress and displace the trachea and compress and stretch the bronchi. Lymphadenopathy is not always easy to detect on conventional radiographs, and CT has higher sensitivity. On enhanced CT, lymph nodes from TB infection may have a characteristic appearance, with central low attenuation and obliteration of perinodal fat. Lymph node calcifications may be seen in 15%.60 Prominent, irregular peripheral enhancement of the enlarged nodes, described as a “ghost-like” pattern of enhancement, has been described.61 Lymphadenopathy from TB can be significant and can coalesce to produce mass-like structures and eventually fibrosing mediastinitis, which is sometimes difficult to differentiate from other soft tissue masses (Fig. 3.31).15 US through a substernal window can be helpful in imaging TB lymphadenopathy, both for diagnosis and for follow-up after treatment in infants and young children. Abnormal lymph nodes are easily distinguished from blood vessels, thymus, and other mediastinal structures62,63 on US in these patients. MRI demonstrates TB lymphadenopathy in the mediastinum and may show low signal in the lymph nodes on T2-weighted MR images, with peripheral enhancement on T1-weighted postcontrast images.33
Histoplasmosis
Histoplasma capsulatum is a dimorphic fungus that causes histoplasmosis, an infectious disease that is endemic to the Ohio and Mississippi River valleys, Central America, northern South America, and some parts of Asia. The spores incite an intense granulomatous reaction in the alveoli, but the infection also spreads into the mediastinal or hilar lymph nodes. Although histoplasmosis is generally an asymptomatic and self-limited disease, it can disseminate in pediatric patients with impaired immunity. Disseminated disease can be a fulminant illness involving multiple organs and producing lymphadenopathy in a similar manner to tuberculosis. Mediastinal histoplasmosis can take the form of enlarged and lobulated granulomatous mediastinal lymph nodes that may cause mass effect on adjacent structures, or manifest as mediastinal fibrosis.64,65
Radiologic manifestations of histoplasmosis are similar to those of TB and maybe subtle on chest radiographs. In addition to pulmonary disease, lymphadenopathy can be also seen. In patients with histoplasmosis infection, mediastinal and hilar lymph nodes are often calcified (Fig. 3.32).64 Crosssectional imaging with CT or MRI often reveals a paratracheal or subcarinal mass with enhancing septa and areas of central necrosis.66 Fibrosing mediastinitis presenting as a paratracheal or subcarinal soft tissue mass with calcifications appears similar to the same entity caused by TB.15
Neoplastic Adenopathy
Lymphoma
Lymphoma is the most common primary neoplasm involving the lymph nodes in the middle mediastinal compartment. However, rather than originating in the middle mediastinum, involvement of this compartment more commonly manifests from extension of an anterior mediastinal mass (Fig. 3.17).1,15,37
Location aside, lymphoma has the same imaging features whether it is in the anterior or middle mediastinal compartment. Chest radiography shows nonspecific mediastinal widening, and cross-sectional imaging is frequently needed for further characterization. Lymphoma typically presents with large homogeneous soft tissue masses on CT or MRI that can cause mass effect on adjacent structures if large enough.1 With increasing size, lymphomatous masses may develop central areas of necrosis and hemorrhage.6,25 Calcification is uncommon before treatment, but not infrequently seen following treatment.37 PET/CT can be obtained for staging and assessment of treatment response.15 Metastatic lymphadenopathy can present in the middle mediastinal compartment, and it is important to note that determining whether
mediastinal lymphadenopathy represents the primary tumor or metastatic disease can be difficult. Correlation with clinical and laboratory parameters is crucial.56
mediastinal lymphadenopathy represents the primary tumor or metastatic disease can be difficult. Correlation with clinical and laboratory parameters is crucial.56
NUT Midline Carcinoma
Nuclear protein in testis (NUT) midline carcinoma is a rare, highly aggressive, highly lethal carcinoma.15,67 These tumors usually arise in midline locations, including the head, neck, and mediastinum. They frequently present in advanced stages of disease and have an extremely poor prognosis.68 Bones are the most common site of extrathoracic metastatic disease.69 Tumor development involves translocation of chromosomes 15 and 19. Resulting abnormal BRD4-NUT and/or BRD3-NUT fusion proteins are thought to repress transcription of genes responsible for squamous differentiation. Lack of differentiation is associated with uncontrolled cellular proliferation. NUT midline carcinoma was initially observed in adolescent and young adult males, although subsequent publications have shown a broader age distribution that ranges from the first decade to the late 8th decade of life.67
The imaging appearance of NUT midline carcinoma is nonspecific and may mimic other mediastinal masses.67 Chest radiographic appearance depends on the size of the lesion, ranging from a focal mass to complete hemithorax opacification.25 CT and MRI show a large, bulky, infiltrative mediastinal and hilar mass, often associated with postobstructive atelectasis and ipsilateral pleural disease (Fig. 3.33).69 MRI features include a heterogeneous mass with predominantly low signal
on T1-weighted sequences and hyperintensity on T2-weighted sequences. There is heterogeneous enhancement, with tumor and lymph node necrosis and hemorrhage, as well as lesional calcification. FDG-PET/CT shows hypermetabolism of the tumor and involved lymph nodes.15,67,68
on T1-weighted sequences and hyperintensity on T2-weighted sequences. There is heterogeneous enhancement, with tumor and lymph node necrosis and hemorrhage, as well as lesional calcification. FDG-PET/CT shows hypermetabolism of the tumor and involved lymph nodes.15,67,68
Variable responses to chemotherapy and radiation therapy have been observed, but outcomes have been extremely poor. Overall survival is relatively improved with early radiotherapy and more extensive tumor resection.37
Castleman Disease
Castleman disease (CD) is an uncommon benign lymphoproliferative disorder.70 The etiology is unknown, but it is believed to be a chronic inflammatory response to viral antigenic stimulation. There are two subgroups: localized (one group of lymph nodes involved) and disseminated (two or more lymph nodal groups involved). Localized disease seems to be much more frequent in the thorax, but CD can be seen in almost any region containing lymphoid tissue. There are three histological subtypes: hyaline vascular CD, plasma cell CD, and plasmablastic CD. Although peak incidence is in the third and fourth decades, CD can present in children, and there is no gender predilection.70,71 Most mediastinal masses are found incidentally, and affected patients are usually asymptomatic. When present, symptoms (e.g., cough, chest pain, and dyspnea) are related to mass effect. Invasion of and adherence to vessels and bronchi are common.71,72 Given its imaging appearance in the mediastinum, it can be mistaken for lymphoma, thymoma, or TB (in endemic areas).72
On chest radiographs, localized CD usually manifests as a mediastinal or hilar mass with smooth margins or lobular contours, whereas disseminated or multicentric CD may result in bilateral hilar and mediastinal enlargement. On CT, it can present as a solitary, noninvasive mass, a mass involving adjacent structures, or matted lymphadenopathy (Fig. 3.34).70 The masses of the hyaline vascular type are extremely hypervascular, showing prompt, homogeneous enhancement, while the masses of the plasma cell type are homogeneous but less enhancing.70,71,72 Irregular central calcifications can be found within the masses.72 On MRI, the lesions are isointense or slightly hyperintense relative to skeletal muscle on T1-weighted sequences, and hyperintense on T2-weighted sequences.71
 FIGURE 3.34. A 10-year-old boy with Castleman disease who presented with intermittent chest pain. Axial enhanced CT image shows a mildly enhancing right hilar mass (arrow). |
There are case reports in which the disease gradually regressed without treatment. When disease is localized, surgical resection is the preferred treatment. For the disseminated form, chemotherapy has been reported to be successful in many cases. Steroids and radiotherapy have previously been used but their role remains uncertain.71
Metastatic Adenopathy
Metastatic adenopathy in the middle mediastinal compartment arises from regional or distant primary sites. The most common adult metastasis is from lung malignancy, such as small cell lung cancer. However, in children, metastatic lymphadenopathy in the middle mediastinal compartment is much less common and usually from primary tumors such as neuroblastoma, Wilms tumor, and sarcoma.1,15,37 There are no established size criteria that differentiate pathologically enlarged from normal-sized lymph nodes in children. Some authors believe that measurable mediastinal lymph nodes in children are always abnormal. The current literature states that lymph nodes are pathologically enlarged if the short-axis dimension measures more than 10 mm in all age groups.15
On chest radiographs, findings of metastatic adenopathy vary depending on the size of the lymph nodes, from normal to a lobulated soft tissue mass within the middle mediastinal compartment. CT or MRI is usually obtained for further evaluation (Fig. 3.35). CT or PET/CT demonstrates single to multiple enlarged, homogeneous soft tissue masses in the middle mediastinal compartment, with homogeneous contrast enhancement and FDG avidity. However, cystic areas reflecting necrosis can be seen. Calcification can be identified in osteosarcoma or radiation-treated lymphoma. As in lymphoma, MRI plays an increasingly prominent role in imaging metastatic lymphadenopathy.1,15
Posterior Mediastinal Lesions
Approximately 30% to 40% of pediatric mediastinal masses are found within the posterior mediastinal compartment.1,37 The majority (88%) of these are neurogenic in origin, arising from mediastinal nervous tissues such as the peripheral nerves, sympathetic ganglia, or paraganglia.1,37 Paravertebral lesions related to infection and trauma may also be encountered in this mediastinal compartment.2 There is a high probability that posterior mediastinal masses may extend into the spinal canal, which warrants detailed imaging evaluation.
Neoplastic Lesions
Sympathetic Chain Ganglion Origin Tumors
Neuroblastoma. In the pediatric population, about 90% of posterior mediastinal tumors are derived from the sympathetic chains located along the thoracic vertebral bodies. Neuroblastoma accounts for the vast majority of these neurogenic tumors.33,56 It is the third most common pediatric cancer and the most common extracranial solid tumor in children. Most are diagnosed before 4 years of age.37,57,73 Neuroblastoma is a malignant tumor of primitive neural crest cells, with the least cellular differentiation of the tumors originating from the sympathetic chain (neuroblastoma,
ganglioneuroblastoma, and ganglioneuroma).73 Although most neuroblastomas arise from the adrenal glands, they can be seen anywhere along the sympathetic chain, particularly within the posterior mediastinal compartment (about 20% of neuroblastomas in the pediatric population).73
ganglioneuroblastoma, and ganglioneuroma).73 Although most neuroblastomas arise from the adrenal glands, they can be seen anywhere along the sympathetic chain, particularly within the posterior mediastinal compartment (about 20% of neuroblastomas in the pediatric population).73
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree