Fig. 1
99mTc-MAG3 dynamic scintigraphy in an adult patient. Images are compound by three different views: functional parameters on the upper left view; renogram curves (red line for the right kidney; green line for the left kidney) on the upper right view; and sequential dynamic images on the lower view, acquired with a 3 s frame for the first 3 min, followed by 10 s frames for the remaining time. The study represents a normal dynamic scintigraphy, with a good split renal function (renogram curves are symmetric). The first images visualize the perfusional phase (up to 1 min), reflecting a rapid increase of the renogram curve. The collecting system and bladder appear in the third to fourth minute, after the activity peak (T max). The excretory phase presents with rapid disappearance of the tracer from the renal parenchyma and drainage through the urinary tract, in keeping with the decreasing part of the renogram (concave line)
Dynamic scintigraphy can be performed either as described above or as a challenging test. The provocative substance can be a diuretic agent, usually furosemide, or an angiotensin-converting-enzyme inhibitor (ACEi), principally captopril. In the first case, the dynamic study is called diuretic scintigraphy and is performed in case of dilated collecting system, query obstruction. The ACEi challenging test is performed in case of high blood pressure, when an underlying renovascular cause is suspected.
Diuretic Scintigraphy
A special mention must be made for diuretic renal scintigraphy. The method comprises a dynamic study performed under diuretic challenging conditions. The aim is the creation of an acute stress to the collecting system, which should help in differing between obstructed and non-obstructed urinary tract. Published guidelines (EANM procedure guidelines) and reported recommendations suggest the use of furosemide as a principal diuretic agent at the dose of 1 mg/kg in infants (<1 year of age), 0.5 mg/kg in children (up to 20 mg) and 40 mg in adults. The furosemide action generally starts 2–4 min after the administration, with maximal effect on diuresis within 15 min (Taylor and Nally 1995).
The validated timing for furosemide can be either 20 min after tracer administration (F + 20), when the maximal tracer retention occurs, or 15 min prior to the injection (F-15), for a maximal diuretic effect during the examination (Hilson 2003).
F + 20 method allows the observation of both unmodified renal handling of the tracer and the effect of an acute diuretic stress, determined by a rapidly increasing urinary flow.
F-15 method (English et al. 1987) includes the administration of furosemide 15 min before the tracer and allows the observation of the urinary tract behaviour under a maximal diuretic effect. The last method to be introduced is F + 0 (Adeyoju et al. 2001). This procedure can be safely performed, as the diuretic does not interfere with the detection of the renal function (Boubaker et al. 2006), and in children, it is preferred for the unique advantage of permitting a single simultaneous injection of tracer and furosemide.
All different methods are interchangeable, because in 90 % of the cases, there is no significant difference in results in between the various timings of furosemide administration (O’Reilly 2003).
Quantitative Methods
Although the “gold standard” for absolute renal function is based on blood sampling, the gamma camera-based methods are sufficiently reliable, even in infants (Piepsz et al. 2005). Dynamic scintigraphy, through a camera-based method, permits the calculation of renal clearance thanks to the fact that early renal uptake is directly related to the renal function. It is also possible to have an estimation of the split renal function (separate renal function) of the kidneys, by calculating the area under the renogram curve, in the initial part of the study, or by calculating the ratio of the slopes over a short period in the uptake phase (Hilson 2003).
There are other accurate and reproducible methods for the renogram analysis (Blaufox et al. 1996), such as the Patlak–Rutland plot (Peters 1994), the deconvolution analysis for parenchymal transit time (Cosgriff and Berry 1982; Lupton et al. 1984), the renal output efficiency (Chaiwatanarat et al. 1993), the pelvic excretion efficiency (Anderson et al. 1997), the normalized residual activity (Piepsz et al. 2000), etc.
Nevertheless, it is worth saying that all known methods used for quantitative evaluation lack accuracy in case of poor renal function (Piepsz et al. 1996).
An overview of the general indications to dynamic scintigraphy includes (a) estimation of renal function through GFR, ERPF and split renal function; (b) urinary tract dilatation/obstruction; (c) renovascular disease; (d) congenital abnormalities; (e) renal transplantation; (f) pre- and postoperative follow-up; and (g) renal trauma.
2.2.3 Static Renal Scintigraphy
The principle of static renal scintigraphy is based on the stable fixation of the radiotracer to the renal parenchyma, permitting the visualization of a distribution map of the functional renal mass, with the possibility to calculate the differential renal function (DRF). Static scintigraphy conceives the acquisition of a single frame image (2–3 h post-injection), usually on the posterior view, but also in other views when necessary, such as anterior or posterior oblique images. Renal and background ROIs are drawn and DRF is estimated by arithmetic or geometric mean on the basis of the revealed radioactivity expressed in k counts.
A possible completion to static scintigraphy is the acquisition of tomographic images through the single-photon emission tomography (SPET) technique. The reconstruction of the data gives way to visual representation of the images in the transaxial, coronal and sagittal planes, but despite special cases, when the 3D vision of the kidneys is necessary, the SPET images do not find a routine utilization in renal nuclear medicine.
Static scintigraphy permits a proper evaluation of the kidneys, by revealing their localization, number, morphology and dimension. This is the case of detection of any possible abnormality, including renal ectopia, dysplasia, hypoplasia, agenesia or any other congenital condition (horseshoe kidneys, cross-fused kidneys, etc.). On the basis of tracer distribution, which should be generally homogeneously fixed in the renal parenchyma, it is possible to detect renal damage visible as areas of abnormal or irregular uptake. This is the case of acute pyelonephritis, renal scars, renal infarction, or other pathologies. Lesions usually present as hypoactive or cold areas, with regional reduction of tracer uptake, mainly located in the renal profile. In extreme cases, parenchymal damage can present with generalized hypofixation and reduced renal volume, up to a shrunken kidney (Figs. 2, 3, 4, 5, and 6).
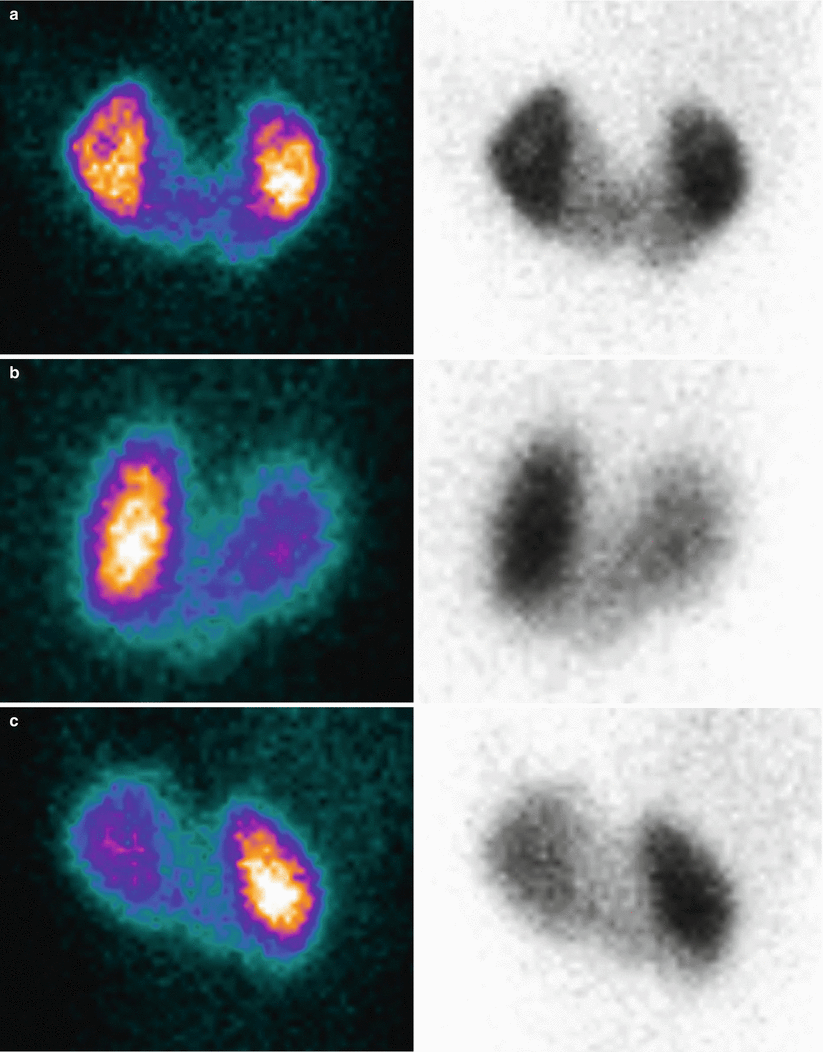
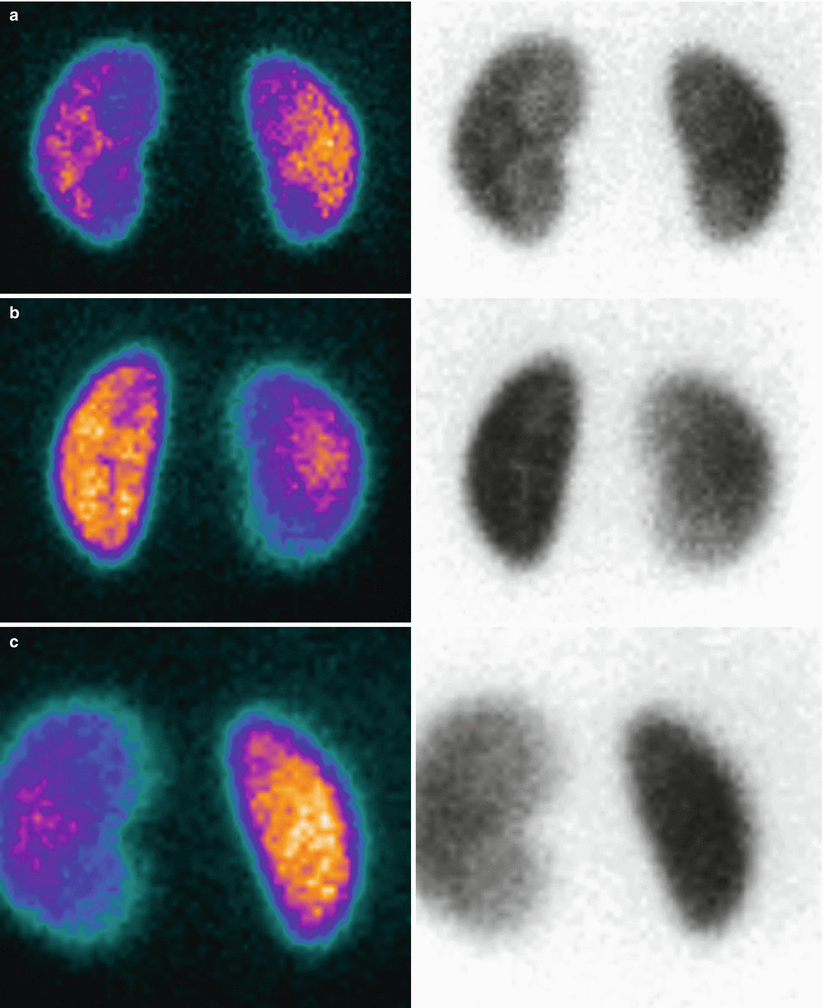
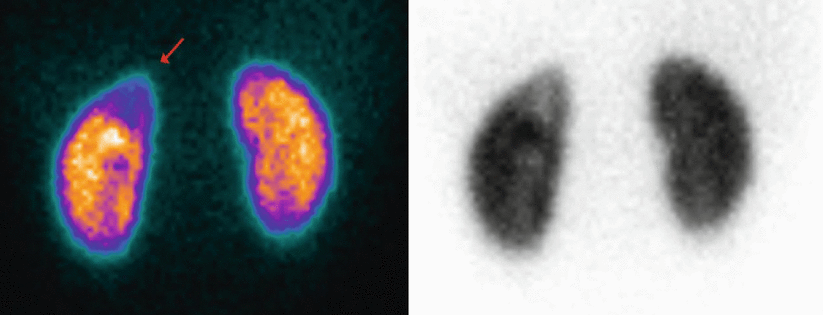
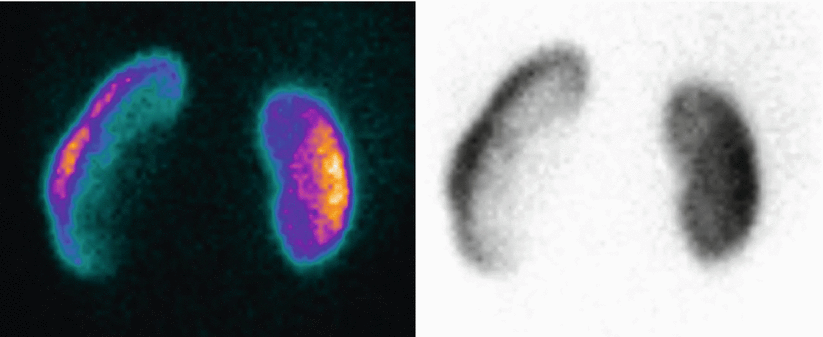
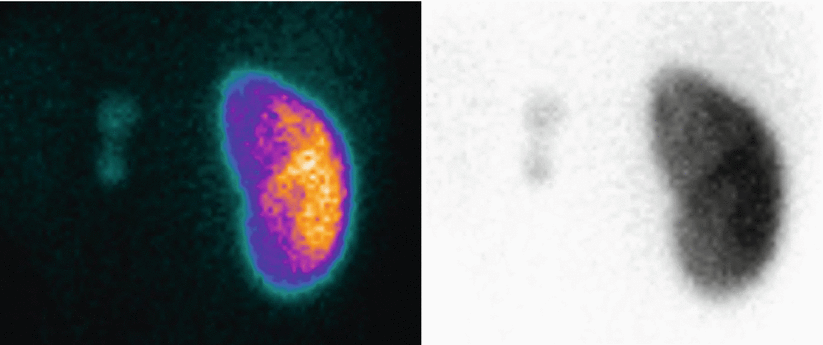

Fig. 2
99mTc-DMSA static renal scintigraphy in an infant of 6 months of age with horseshoe kidneys. Images are represented in colour (warm metal scale) and in black and white (inverted grey scale). DRF is 49.8 % for the right kidney and 50.2 % for the left kidney. (a) Posterior view; (b) posterior left oblique view; (c) posterior right oblique view

Fig. 3
99mTc-DMSA static renal scintigraphy in a 5-year-old child. Images are represented in colour (warm metal scale) and in black and white (inverted grey scale). DRF is 53.3 % for the right kidney and 46.7 % for the left kidney. (a) Posterior view; (b) posterior left oblique view; (c) posterior right oblique view. The study reveals indirect signs of bilateral pyelectasis with cortical thinning mainly in the polar regions

Fig. 4
Posterior view of 99mTc-DMSA static scintigraphy in a 7-year-old child. DRF is 50.5 % for the left kidney and 49.5 % for the right kidney. The patient presented a UTI at birth. VCUG showed no VUR and the child was referred for a follow-up static scintigraphy. Both images show a markedly reduced uptake of the tracer in the upper pole of the left kidney (arrow) as a result of cortical damage (renal scarring)

Fig. 5
Posterior view of 99mTc-DMSA static scintigraphy in an 8-year-old child. DRF is 55.8 % for the right kidney and 44.2 % for the left kidney. The patient had a prenatally diagnosed left hydronephrosis, resulting in an obstructed PUJ. During the first year of age, the child underwent corrective surgery with Anderson Hynes pyeloplasty. Follow-up static scintigraphy shows a large left kidney with thinned renal parenchyma stretched round the intrarenal collecting system, which is still dilated, but no longer obstructed. The right kidney shows some cortical thinning in the polar regions, more marked on the upper pole, and indirect signs of slightly dilated intrarenal collecting system

Fig. 6
Posterior view of 99mTc-DMSA static scintigraphy in an 8-year-old child. DRF is 97 % for the right kidney and 3 % for the left kidney. The patient presents a prenatally diagnosed hypoplastic left kidney, severely damaged in the intrauterine period. On static scintigraphy, the left kidney presents as a slightly visible area of very small dimensions. The right kidney shown is increased in dimensions, with good functional development and indirect signs of dilated collecting system, especially in the polar regions, secondary to functional compensation
An overview of the principal indications for static scintigraphy includes (a) acute pyelonephritis, (b) estimation of renal scarring, (c) determination of functional renal mass and DRF, (d) congenital abnormalities and (e) post-renal transplantation.
2.2.4 Radionuclide Cystography
Radionuclide cystography (RNC) is the nuclear medicine alternative to voiding cystourethrography (VCUG). As its radiological relative, RNC focuses on the detection and qualification of physiological and anatomic abnormalities of the genitourinary system by producing images of diagnostic quality.
The technique contemplates two possible approaches. The first is the direct radionuclide cystography (DRC), which, similar to VCUG, requires catheterization of the bladder and instillation of radionuclide and fluids for a maximal distension of the bladder. Images are then acquired from the filling of the bladder to the voiding and post-voiding phases. The tracer utilized can be 99mTc-sodium pertechnetate, sulphur colloid or DTPA, with a very low administered activity (round 37 MBq). The amount of liquids to introduce in the bladder is usually calculated through age-related formulas, which differ whether the patient is an adult or a child (EANM procedure guidelines).
Main drawbacks of DRC are related to invasiveness, similar to VCUG, and non-physiological setting, as the bladder is forcedly filled and the urinary flow does not follow natural course.
The other alternative for RNC is called indirect radionuclide cystography (IRC), which does not require bladder catheterization, but generally follows a dynamic scintigraphy, with an IV injection of the radiopharmaceutical (mainly MAG3 or DTPA). The main limitation of IRC is the necessity of toilet-trained patients. Principally, RNC is used to evaluate vesicoureteral reflux, with comparable sensitivity but with less gonadal radiation, when compared with conventional radiological technique (VCUG). RNC, however, does not provide the same anatomic details, and this is the reason why it is not the first-line investigation in males.
In both cases, images are acquired in the posterior view, with a framing rate of 10–30 s per frame. Specific ROIs are drawn over the bladder and in the renal regions, for the elaboration of time/activity curves. Post-micturition residual volumes are quantified with a delayed image post-voiding.
Common indications for a RNC, as reported by approved guidelines (EANM procedure guidelines), include initial evaluation of females with urinary tract infection (UTI) for reflux, diagnosis of familial reflux, evaluation of vesicoureteral reflux after medical management, assessment of the results of antireflux surgery and serial evaluation of bladder dysfunction for reflux.
3 Clinical Applications
Nuclear medicine investigations hold a significant diagnostic role in clinical practice thanks to the remarkable information they provide. These information include the possibility to study the entire excretory system, including the kidneys and urinary tract, to obtain functional data and quantify them and at last to perform challenging tests.
An integrated and optimal diagnostic approach must also take into account other potentialities and peculiarities, which render functional investigation generally advantageous. First of all, they are easily feasible, relatively cheap and noninvasive procedures, conceiving generally IV injections. The maximum invasiveness is represented by bladder catheterization in case of DRC. Nuclear medicine investigations utilize radioactive substances, but the radiation burden to which patients are submitted is objectively low. An overview of the effective doses during the main examinations performed documents 0.5–0.7 mSv for a static 99mTc-DMSA scintigraphy and 0.3–0.6 mSv for a dynamic 99mTc-MAG3 scintigraphy (Fanti 2003). These values are restrained if we compare them with other imaging techniques, such as renal angiography (5–7 mSv) or TC (10 mSv).
In addition, nuclear medicine examinations are easily reproducible. The possibility to safely and reliably repeat an examination, without the risk of main discrepancies, has main relevance in clinical practice, especially in those conditions requiring a functional comparison before and after a therapeutic approach or in case of long-term follow-up.
These characteristics render nuclear medicine investigations largely used in renal disorders, with main indications on detection, evaluation and quantification of renal function, urinary tract dilatation, UTI, renovascular disease or renal transplantation. However, any potential congenital and acquired condition may benefit from a functional investigation with a renal nuclear medicine examination.
3.1 Renal Failure
The evaluation of renal function represents an important part of the investigations performed in nuclear medicine. The methods utilized, either based on blood sampling or camera based, are accurate and relatively simple to perform. There exists, however, a proper indication for nuclear medicine examinations in case of deterioration of renal function. Renal failure can be either an acute or a chronic impairment of the excretory function and, in an overwhelming number of cases, can be diagnosed by a specific renal scintigraphy (Fig. 7).
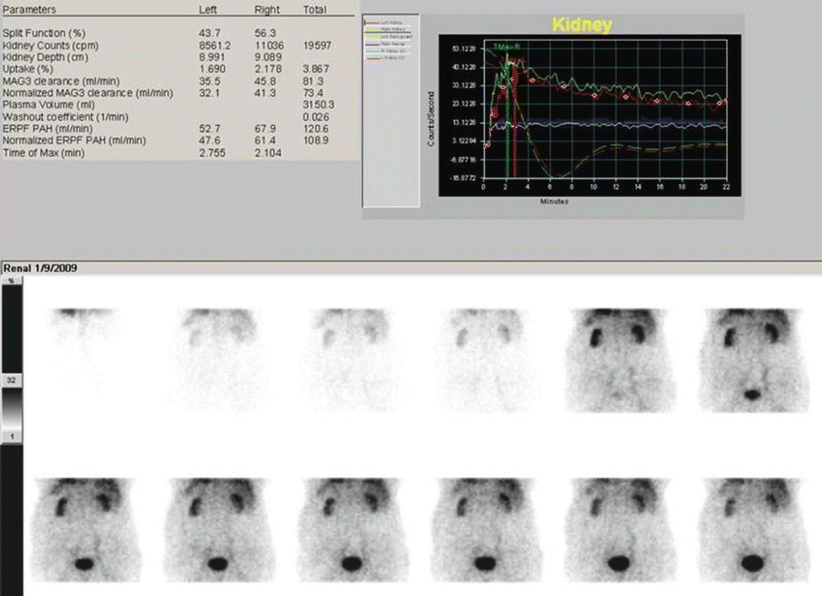

Fig. 7
99mTc-MAG3 dynamic scintigraphy in an adult patient with chronic renal failure (serum creatinine is 3.8 mg/dL). Renal impairment is symmetric and can be immediately evidenced thanks to the reduced renal dimensions and the high radioactivity seen in the background. The tracer uptake is modest, bilaterally, as confirmed by the reduced amplitude of the renogram curves. The reduced vis a tergo determines, in addition, a slower urinary excretion
When the process develops slowly under a chronic cause, or simply as a consequence of physiological decline with age, renal failure can be difficult to reveal and its real gravity is easily underestimated. The routine examination is serum creatinine, but it requires an important loss of functioning parenchyma to become abnormal and can remain within the normal range despite an inulin clearance of 60–80 % below normal values (Levey et al. 1991).
In this context, renal scintigraphy has a proper indication in the quantification of chronic renal impairment, thanks to the more reliable proportion existing between clearance estimated by nuclear medicine methods and renal function. The strength of nuclear medicine in renal impairment is its ability to assess renal function even in case of very low levels of clearance.
Differently from chronic renal failure, whose reversibility is considered unlikely and therapeutic intervention is mainly limited to the prevention of further progression, in acute renal failure, there exists the real possibility of reversibility and, hence, recovery of the function.
Nuclear medicine has no utility in the immediate assessment of acute renal failure, as the priority in the initial phases is the rapid recognition of this life-threatening condition and recovery of the reversible factors.
Acute renal failure is often multifactorial and its causes are usually classified as prerenal, renal and post-renal. Imaging in acute renal failure has two potential roles, diagnostic and prognostic.
Prerenal causes of acute failure are usually dehydration or shock, which when prolonged in time may precipitate in acute tubular necrosis (ATN). It is not exactly a necrosis and represents temporary and reversible cessation of renal function. ATN presents an initial phase with oliguria, followed by a progressive improvement of renal function over 7–14 days manifested with a polyuric phase (Hilson 1994). As the perfusion is generally maintained in the initial phase, the typical nuclear medicine appearance in dynamic scintigraphy would be well-vascularized kidneys, but with absent or very poor tracer concentration or excretion. With 99mTc-DTPA, we can see a good vascular pool in the first pass, but no tracer retention and no visualization of the collecting system. With tubular excreted agents (MAG3), the feature changes and dynamic scintigraphy reveals a progressive tracer uptake in the kidneys, with no evidence of excretion.
A prognostic estimation can be done on the basis of the data derived from tubular excreted agents. If tubular function is preserved, typically seen as progressive accumulation of the tracer, the prognosis is generally good. But even if tracer retention is not seen, it does not mean that there will not be any recovery (Hilson 1994).
When the cause of renal failure is a parenchymal disease, nuclear medicine role is somehow limited, as despite giving a value to renal impairment, it has nothing to offer in identifying the specific renal pathology. Size and function criteria are not exclusive for one disease or another. In addition, some parenchymal pathologies, such as glomerulonephritis, diabetes, amyloidosis or myeloma, may present normal-sized or even enlarged kidneys, with globally reduced function. In these cases and in case of an acute deterioration in chronic failure, nuclear medicine finds a proper indication in a prognostic vision. A reasonably well-perfused kidney suggests potential recovery, although there will be a return to normal function only if before the onset of the acute failure the kidneys were normal.
The less frequent cause of acute renal failure is connected to post-renal problems, almost exclusively due to obstruction, which is one of the reversible causes of renal failure (O’Reilly et al. 2001). The drainage problem must be either bilateral or occurring in a solitary kidney; otherwise, it is unlikely to reach renal impairment by a simple obstruction, as the contralateral kidney usually compensates. Although obstruction is generally detected by a dynamic scintigraphy, this is not always the case, as for severe obstruction there can be a potential renal functional exclusion. In these cases, diagnosis must be a combination of different imaging techniques for the clinical manifestation.
3.2 Urinary Tract Dilatation
In clinical practice, one of the principal requests for a nuclear imaging study concerns urinary tract dilatation. The main problem to investigate in urinary tract dilatation is the presence of obstruction, which when left untreated can lead to the loss of renal function. In adults, urinary tract dilatation must always be considered pathologic, as the cause, in an overwhelming number of cases, is an obstruction, or less frequently, a consequence of congenital abnormalities, left undiagnosed. In children, urinary tract dilatation can be related to many different pathological or non-pathological entities, which can not only include exclusively obstruction but either vesicoureteric reflux (VUR) or other paraphysiological situations, although the most worrisome condition is an obstructive system.
Presentation and diagnosis are different whether the patient is an adult or a child. In adults, obstruction is more frequently referred to a chronic condition, leading to flank pain, renal lithiasis, UTI or a progressive renal deterioration. As an acute event, it is usually a result of urinary stones or, more rarely, a consequence of iatrogenic cause (intervention, compressive masses, etc.). In paediatric population, obstruction is a complex clinical entity, mainly determined by congenital defects concerning potentially any part of the urinary tract (pelviureteric junction, vesicoureteric junction, urethra, etc.). One of the most frequent causes of obstruction in children is related to a unilateral or bilateral hydronephrosis. The pathology can be diagnosed either in the intrauterine period, by means of prenatal ultrasonography, or during childhood. In the later case, clinical presentation may be similar to the adult symptoms, but in some cases, the pathology can be completely silent.
Nuclear medicine can make an important contribution in discriminating between obstructed and non-obstructed system, as well as assessing the functional and urodynamic results of a corrective surgery (O’Reilly et al. 1978, 2001). The procedure of choice is diuretic scintigraphy which, compared to other imaging techniques, is essential in distinguishing patients who can benefit from surgery from those who can be treated conservatively (Boubaker et al. 2006; O’Reilly 2003).
In diuretic scintigraphy, the main drainage patterns to take into account can be classified as normal, dilated non-obstructed, equivocal and obstructed pattern.
In adults, referring parameters to assess obstruction are time to activity peak (T max) and half-time or time to obtain a 50 % of the tracer washout (T1/2).
For the interpretation of data, either a visual analysis or a quantitative evaluation can be used. The visual analysis takes into account the aspect of the sequential dynamic images, produced by the summarized frames, but also the trend of the washout curves, rather than the rate of washout by measuring the T1/2 after furosemide. Prompt clearance of the radiopharmaceutical from the renal pelvis with a T1/2 of less than 10 min (up to 15 min) is considered a normal response, while a T1/2 greater than 20 min suggests obstruction (Figs. 8 and 9). Values between 15 and 20 min are considered indeterminate and should be interpreted with the context of the examination (O’Reilly et al. 1978). Approximately 10–17 % of the studies will be difficult to interpret because of an indeterminate response (Taylor and Nally 1995).
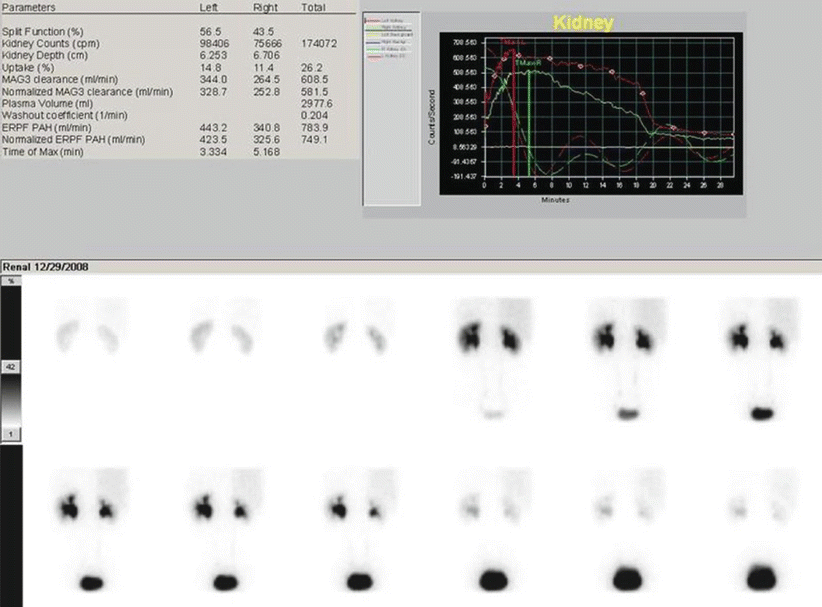
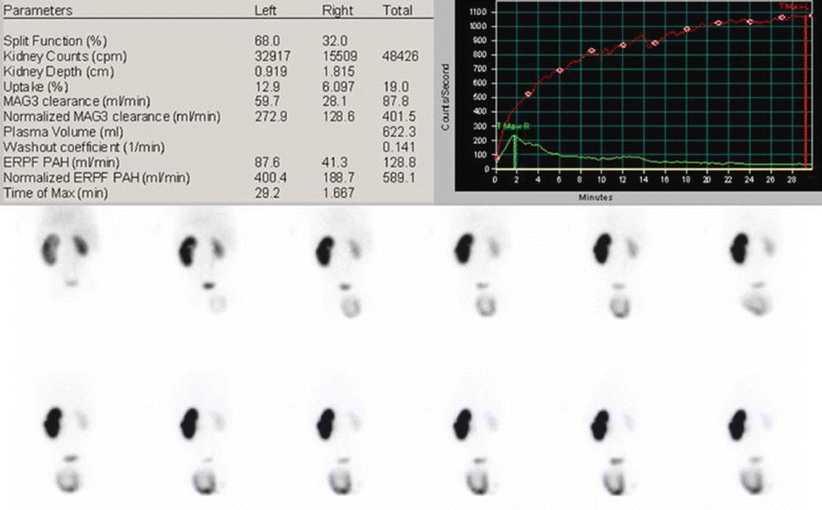

Fig. 8
99mTc-MAG3 diuretic scintigraphy in an adult patient with bilateral pelvic dilatation, query obstruction. Images are acquired for 30 min, with the possibility of diuretic administration during the study. Both kidneys show a good tracer uptake, less prominent in the right kidney. The excretory phase is partially delayed, especially on the left kidney. Furosemide was given at 15 min after tracer injection. Sequential summarized frames show a significant washout of the tracer from the collecting system after the diuretic effect. Renogram curves confirm a prompt response to stimulated diuresis, by showing a rapid stepwise decrease in radioactivity. This sort of response is in keeping with dilated non-obstructed collecting systems

Fig. 9
99mTc-MAG3 diuretic scintigraphy in a 3-month-old infant with left pyelectasis, query PUJ obstruction. When compared to the contralateral, the left kidney appears larger. The tracer uptake is markedly increased, with no sign of left drainage even after the diuretic effect. Furosemide was administered simultaneously to the tracer (F + 0). The right kidney has a normal activity, as demonstrated by the corresponding renogram, with a slightly evident stepwise response to furosemide at the 15th minute. On the left, there is a gigantic curve, with progressive increase in activity. This feature is a result of a well-compensated PUJ obstruction
Usually, in concordance with the above-described features, the corresponding renograms show indicative shapes. The washout curve for a non-obstructed kidney is more likely of concave shape, whereas the washout curve for an obstructed kidney is usually a rising or convex curve. All the other shapes, not included in obstructed or non-obstructed cases, can be considered equivocal.
Some conditions, however, may lead to stepwise or irregular curves depending on other conditions, such as large fluctuations in blood flow, VUR, double moiety or intermittent hydronephrosis. Intermittent hydronephrosis may occur as a delayed double peak curve and can be considered as a possible fifth drainage pattern. It occurs usually 10–15 min post-diuretic injection and presents with an initially rapid tracer elimination followed by a sudden cessation of the effect or reversion to a rising curve. This type of response is known as Homsy’s sign (Homsy et al. 1988) and is an indicator to an intermittent hydronephrosis. To fully clarify the pattern, an F-15 study is indicated, as in selected cases it will lead to normalization of equivocal studies (Taylor and Nally 1995).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree