Metabolic brain disorders generally result from a disturbance of normal metabolic processes involving defects in the synthesis or breakdown of certain substances. Clinical manifestations are usually based on the site of the disturbance and the age at which it occurs. Other organs besides the brain are commonly affected, underscoring the importance of an holistic approach to patient evaluation.
Metabolic disorders of the CNS can be classified pathogenetically as follows:
Mitochondrial disorders.
Peroxisomal disorders.
Lysosomal disorders.
Golgi apparatus disorders.
It is often difficult to correlate MRI findings with pathogenesis. For simplicity, therefore, we subdivide the diseases in this chapter into three main groups based on pattern of involvement, while limiting our attention to the most common of these (generally rare) disorders:
Disorders primarily affecting the white matter.
Disorders primarily affecting the gray matter.
Disorders affecting both the gray and white matter.
Pitfall
The differentiation of metabolic disorders by pattern of involvement is often valid only in the early stage and becomes less precise as the disease progresses over time. By the late stage, an accurate classification cannot always be made based on morphologic imaging criteria alone.
7.2 Magnetic Resonance Imaging in Metabolic Brain Disorders
The following imaging sequences are recommended as a basic MRI protocol for investigating a presumed metabolic disorder of the CNS:
T2w sequence in at least the axial and sagittal planes; coronal views may be added if required.
Axial FLAIR sequence.
Axial T1w sequence before and after intravenous contrast administration.
Diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) mapping.
Diffusion tensor imaging (DTI) sequence, if required.
Magnetic resonance spectroscopy (MRS).
Of course, these sequences can also be acquired and reconstructed as three-dimensional images, depending on the scanner.
7.2.1 Diffusion-Weighted MRI
Various types of edema can be distinguished in DWI:
Cytotoxic edema: This type results from a breakdown of cellular energy metabolism. Signal intensity on DWI is increased while the ADC is decreased.
Vasogenic edema: This type is caused by a disruption of the blood-brain barrier. Signal intensity on DWI is decreased while the ADC is increased.
Myelin edema: Myelin edema results from a myelin disorder. As with cytotoxic edema, it is associated with increased DWI signal and decreased ADC.
7.2.2 Magnetic Resonance Spectroscopy
The metabolites that can be differentiated by proton MRS (1H spectroscopy) include the following:
N-acetylaspartate: A marker for intact, viable neurons. The N-acetylaspartate peak is generally reduced in response to neuronal injury.
Creatinine/phosphocreatine: Could play a role in energy metabolism.
Choline: A marker for membrane metabolism. The choline concentration is elevated in response to inflammatory and neoplastic processes.
Lactate: A marker for anaerobic metabolism. Normally it is not present but is elevated in response to mitochondriopathies. Note, however, that the presence of a lactate peak is not specific for mitochondriopathies.
7.3 Normal Myelination in Children
Normal gross myelination, which is marked by corresponding signal changes in T2w and T1w sequences, occurs mainly in the first 24 months of life and follows a predictable timeline.
Note
It is important to know the chronological order of normal myelination in order to distinguish pathologic changes from physiologic processes.
As myelination proceeds, the white matter becomes increasingly hyperintense on T1w images and more hypointense on T2w images ( ▶ Fig. 7.1). Compared with the adult brain, the unmyelinated zones appear “darker” on T1w images and “brighter” on T2w images.

Fig. 7.1 Normal myelination pattern. Axial T2w images in a 7-month-old girl. (a) Myelination of the pericentral region with a corresponding decrease in T2w signal intensity. (b) Myelination of the anterior and posterior limbs of the internal capsule. (c) Myelination of brainstem structures and the central portions of the cerebellar hemispheres.
When MR images of the pediatric neurocranium are interpreted, it should always be determined whether the myelination pattern is normal for age. If myelination is found to be abnormal, the next step is to determine whether the abnormality should be classified as dysmyelination, demyelination, or hypomyelination. It is also important to determine whether myelination is merely delayed (and will “catch up” over time) or arrested.
T2w images in a mature newborn will normally show myelination of the posterior limb of the internal capsule, posterior pons, and central portions of the cerebellar hemispheres. As maturation continues, myelination spreads to the pericentral region, to the anterior limb of the internal capsule, and then anteriorly and posteriorly from that region. Finally the subcortical U-fibers and temporal lobe are myelinated. Gross myelination should be complete on T2w and T1w images by 2 years of age.
7.4 Metabolic Disorders Primarily Affecting the White Matter
White-matter diseases can be divided into two main categories:
Diseases caused by a congenital myelin disorder leading to the defective formation, destruction, or shortened life cycle of myelin.
Diseases of myelin that is normally formed initially.
As a general rule, symmetrical white-matter signal changes indicate a disease in category 1, i.e., a congenital metabolic disorder. Asymmetrical changes, on the other hand, are more consistent with a category 2 disease, i.e., an acquired disease of normally formed myelin. This section deals mainly with category 1 diseases, which are true metabolic disorders of the white matter.
Various aspects should be considered in the differentiation of white-matter diseases:
Imaging morphology: Particular attention is given to distribution pattern, possible contrast enhancement, and changes in diffusion properties and MRS.
Age: How old was the patient at disease onset?
Clinical presentation: Does the patient show signs of motor, cognitive, or behavioral impairment? Are other organs involved? Check for cataract, hepatomegaly, etc.
Rate of disease progression: How rapidly is the disease progressing?
Neurophysiologic tests: For example, evoked potentials.
Laboratory tests: For example, lactate elevation.
Genetic causes: Genetic test results.
The interpretation of MRI findings in white-matter metabolic disorders can be a difficult task. For initial orientation, it is helpful to evaluate the pattern of spread of the signal changes:
Posterior to anterior: One possibility is X-linked ▶ adrenoleukodystrophy.
Anterior to posterior: Potential causes include ▶ Alexander’s disease .
Inside to outside: This pattern would be consistent with ▶ metachromatic leukodystrophy, for example.
Outside to inside: This pattern would be consistent with ▶ glutaric acidurias, for example.
It is also important to consider or elicit clinical parameters. For example, the hypotonic muscles of a “floppy infant” suggest a very different disorder than spasticity. Pronounced hypotonia would be consistent, for example, with merosin-deficient leukodystrophy, which presents with congenital muscular dystrophy.
The clinical course is also extremely important in classifying leukodystrophies. Some diseases are rapidly progressive while others take a slow course. There are also diseases in which the patient’s condition may worsen during periods of “metabolic crisis.”
Age at onset is another important parameter. Most leukodystrophies begin in childhood or adolescence, with earlier onset usually indicating a poor prognosis.
7.4.1 Leukodystrophies Primarily Affecting the Deep White Matter
7.4.1.1 Adrenoleukodystrophy
Classic (X-linked) adrenoleukodystrophy is a disease that is inherited through the X chromosome, occurs predominantly in boys, and has a prepubertal onset. There are also special forms, but our attention here is limited to just one of these: adrenomyeloneuropathy. It is also inherited via the X chromosome, and heterozygous women may show relatively mild neurological impairment. Adrenomyeloneuropathy has a later onset than X-linked adrenoleukodystrophy and may develop during puberty or even at an older age. Spinal and cerebellar involvement are predominant in this disease, producing clinical symptoms such as paraparesis or cerebellar dysfunction. The disease may be on a continuum with X-linked adrenoleukodystrophy.
Pathology Adrenoleukodystrophies are disturbances of peroxisomal metabolism that interfere with the β-oxidation of very-long-chain fatty acids. It is characterized by a rapid course and generally poor prognosis.
Clinical manifestations The most common clinical manifestations of adrenoleukodystrophies are hyperpigmentation of the skin, behavioral changes, hearing loss, and visual and balance disturbances.
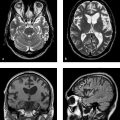
MRI findings MRI in the early phase of adrenoleukodystrophy shows symmetrical changes involving the splenium that spread first to the peritrigonal white matter and then to the occipital white matter. The affected white matter shows markedly decreased signal intensity in T1w images and increased signal intensity in T2w images ( ▶ Fig. 7.2). Contrast enhancement typically occurs at the periphery of the lesions (see ▶ Fig. 7.2c). Three zones, called Schaumburg zones, can usually be identified in affected white matter:
An inner zone of complete demyelination.
An adjacent zone of inflammation.
An outer zone of active demyelination.

Fig. 7.2 Adrenoleukodystrophy in a 6-year-old boy. (a) Coronal FLAIR image shows marked hyperintensity in the parieto-occipital and peritrigonal regions. (b) Sagittal T1w inversion-recovery image displays the various zones of myelin destruction. (c) Coronal T1w image after contrast administration shows definite enhancement at the periphery of the demyelinated areas.
Generally the peripheral white matter is affected at a relatively late stage. The subcortical U-fibers are usually spared. DWI shows restricted diffusion in the inflammatory zone. Images of the brainstem region usually show bilaterally symmetrical involvement of the pyramidal tracts.
Note
Adrenoleukodystrophy typically spreads in a posterior-to-anterior pattern. The zone of inflammation shows contrast enhancement and restricted diffusion.
7.4.1.2 Metachromatic Leukodystrophy
Pathology Metachromatic leukodystrophy is a lysosomal storage disease characterized by a deficiency of the enzyme arylsulfatase A.
Clinical manifestations and epidemiology Three clinical forms of metachromatic leukodystrophy are distinguished by age at onset:
Late infantile form. This form is the most common and has the poorest prognosis. The late infantile form usually presents after 2 years of age with gait disturbance, strabismus, ataxia, and general weakness with rapid progression and death within a few years.
Juvenile form: The less common juvenile form presents at approximately 5 to 10 years of age with declining school performance and mental deterioration. Spastic paralysis eventually supervenes. Progression is slower than in the infantile form.
Adult form: This form begins at 20 to 40 years of age and progresses more slowly than the other two forms. Patients often present with progressive dementia and possible symptoms of schizophrenia and other psychiatric disorders. An important differential diagnosis is multiple sclerosis.
The overall incidence of metachromatic leukodystrophy is approximately 1:100,000, and both sexes are affected equally.
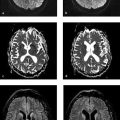
MRI findings T1w MRI in the early stage generally shows decreased signal intensity in the deep white matter. Initial changes typically appear in the peritrigonal white matter ( ▶ Fig. 7.3); early involvement of the cerebellar hemispheres is also common. T2w sequences sometimes show absence of signal changes in the white matter around the transmedullary vessels (and thus around the Virchow–Robin spaces). This may create a tiger-stripe or leopard-skin pattern ( ▶ Fig. 7.4), depending on the image plane. The subcortical U-fibers are generally spared in the early stage. As the disease progresses, the signal changes spread to the more peripheral white matter and atrophy develops. This stage is characterized by progressive involvement of the subcortical U-fibers, corpus callosum, and pyramidal tracts. Cases with onset at a later age often show white-matter changes with an initial frontal predominance. In contrast to ▶ adrenoleukodystrophy, white-matter enhancement does not occur in metachromatic leukodystrophy.

Fig. 7.3 Metachromatic leukodystrophy in a 2-year-old girl. (a) Peritrigonal hyperintensities in an axial T2w image. (b) Peritrigonal hyperintensities in a sagittal T2w image.

Fig. 7.4 Metachromatic leukodystrophy in a 7-year-old boy. The typical tiger-stripe or leopard-skin pattern is visible in certain planes. (a) Axial T2w image. (b) Sagittal T2w image. (c) Axial T1w inversion-recovery image.
Note
Metachromatic leukodystrophy produces a butterfly-shaped pattern of signal changes in the deep white matter of the hemispheres and periventricular white matter. A tiger-stripe pattern is also common due to the relative sparing of perivascular myelin around the Virchow–Robin spaces.
7.4.1.3 Krabbe’s Disease (Globoid Cell Leukodystrophy)
Pathology and epidemiology Krabbe’s disease is a sphingolipidosis caused by a deficiency of the lysosomal enzyme galactocerebroside β–galactosidase. There are infantile, juvenile, and adult forms, and both sexes are affected equally. The incidence is approximately 1:100,000 in the general population. The incidence in the Druze population (originating in south-west Asia) is considerably higher, at approximately 6:1000.
Clinical manifestations The early form of Krabbe’s disease usually presents in newborns with irritability and hypersensitivity to external stimuli. Affected infants may also show feeding difficulties and failure to thrive, and seizures may develop over time. Most cases show rapid progression and a poor prognosis. Very few patients with early-onset Krabbe’s disease survive past 2 years of age. If the onset of disease is in adolescence or adulthood, progression tends to be slower. Affected patients often develop blindness, cerebellar ataxia, spastic paralysis, and peripheral polyneuropathy.
CT findings CT usually shows faint hyperdensities in the thalamus, cerebellar white matter, and corona radiata.
MRI findings T1w images in newborns often show hyperintensities in the thalamus, caudate nucleus, and corona radiata. T2w images initially demonstrate patchy hyperintensities in the deep white matter, which later coalesce. They usually show a symmetrical distribution. The subcortical white matter is still unaffected in the early stage of the disease. A tiger-stripe pattern may be present as in ▶ metachromatic leukodystrophy. The changes spread in a posterior-to-anterior direction, usually starting in the parietal lobes. The optic nerve (including the chiasm) and cranial nerves are usually thickened and may show increased enhancement. White-matter hyperintensities are also seen in the cerebellum, often with subtle rounded or ringlike signal changes in the region of the dentate nucleus ( ▶ Fig. 7.5). DTI of the corticospinal tracts may show decreased fractional anisotropy.

Fig. 7.5 Krabbe disease in a 6-month-old girl. Axial T2w image shows increased signal intensity in the region of the dentate nuclei.
Note
Metachromatic leukodystrophy produces a butterfly-shaped pattern of signal changes in the deep white matter of the hemispheres and periventricular white matter. A tiger-stripe pattern is also common due to relative sparing of the perivascular myelin around the Virchow–Robin spaces.
7.4.1.4 Merosin-Deficient Congenital Muscular Dystrophy
Pathology Merosin-deficient congenital muscular dystrophy is caused by a deficiency of merosin in the muscle fibers.
Clinical manifestations Decreased intrauterine movements are frequently noted before birth. Newborns often show marked hypotonia (“floppiness”), and multiple joint contractures (arthrogryposis multiplex) may be present. Most affected children have normal intelligence.
MRI findings MRI shows pronounced hypomyelination of the white matter ( ▶ Fig. 7.6). As a rule, the deep white matter is predominantly affected. The subcortical U-fibers and internal capsule are usually spared. In rare mixed forms, cerebral malformations and mental retardation may additionally be present.

Fig. 7.6 Merosin-deficient congenital muscular dystrophy in an 8-year-old girl. Axial T2w image shows diffuse hyperintensity of the deep white matter. The subcortical U-fibers are spared.
7.4.1.5 Homocystinuria (Hyperhomocysteinemia)
Pathology and epidemiology Homocystinuria, also called hyperhomocysteinemia, is one of a group of disorders characterized by a disturbance of methionine metabolism, leading to an abnormal accumulation of homocystine in the body. The incidence is approximately 1:24,000 to 1:260,000.
Clinical manifestations The clinical presentation is highly variable. Affected children generally appear normal at birth. Later they show delayed motor and cognitive development, with early manifestations of cerebral ischemia. Epileptic seizures are common. Ocular examination will very often show a characteristic dislocation of the lens. Patients may also have arterial and venous coagulation disorders with thrombus formation.
Note
Patients with homocystinuria are at increased risk for cerebrovascular ischemic events in childhood.
MRI findings The primary changes of homocystinuria usually involve the skeleton and limbs. MRI of the neurocranium often shows only evidence of prior ischemic processes and thrombosis. Dural calcifications may also be present.
7.4.1.6 Maple Syrup Disease
Pathology and epidemiology Maple syrup disease is a branched-chain disease characterized by a disturbance of amino acid metabolism. The average incidence is approximately 1:850,000; it is considerably higher among Ashkenazi Jews.
Clinical manifestations and treatment This rare disease is usually detected early by neonatal screening, and patients have a good overall prognosis with early initiation of treatment. Failure to provide early treatment may lead to life-threatening ketoacidosis, elevated ammonia levels, and permanent brain damage as early as the first or second week of life. Patients often present clinically with poor feeding, vomiting, and lethargy. The urine typically has a distinctive maple syrup odor.
MRI findings MRI usually shows typical edema of the deep cerebellar hemispheres, brainstem, and posterior limb of the internal capsule. The edema is accompanied by markedly restricted diffusion in areas already myelinated at birth.
Note
Untreated maple syrup disease leads to typical edematous zones in the deep cerebellar hemispheres, brainstem, and internal capsule, which are the zones of early myelination. The prognosis depends critically on making an early diagnosis.
7.4.1.7 Phenylketonuria
Pathology and epidemiology Phenylketonuria is a disturbance of amino acid metabolism in which the breakdown of phenylalanine to tyrosine is impaired. It is one of the most common congenital metabolic disorders, and its incidence may vary greatly in different geographic regions.
Clinical manifestations and treatment The full-blown clinical presentation of phenylketonuria is rarely witnessed today as a result of neonatal screening. Successful treatment relies on early diagnosis and lifelong dietary therapy. Untreated, the disease leads to delayed physical and mental development with cognitive dysfunction and epileptic seizures.
MRI findings MRI shows abnormal myelination of the white matter in T2w sequences, with initial changes usually appearing in the deep white matter.
7.4.1.8 Lowe’s Syndrome
Pathology Lowe’s syndrome, also known as oculocerebrorenal syndrome, is a very rare X-linked recessive disorder.
Clinical manifestations Patients present with congenital cataracts in both eyes, impaired renal function like that in De Toni–Fanconil syndrome, and associated growth disturbances. Muscle hypotonia is also evident at birth, and most affected children show variable degrees of mental retardation.
MRI findings MRI usually demonstrates confluent leukodystrophic signal changes in the deep white matter along with white-matter cysts that are isointense to cerebrospinal fluid (CSF).
7.4.2 Leukodystrophies Primarily Affecting the White Matter
7.4.2.1 Megalencephalic Leukoencephalopathy with Subcortical Cysts (Van der Knaap’s Disease)
Pathology Megalencephalic leukoencephalopathy is a rare, autosomal dominant disorder characterized by macrocephaly and the subsequent development of white-matter swelling and typical subcortical cysts. Most cases are caused by a mutation of the MLC1 gene on chromosome 22q13.33.
Clinical manifestations Affected children usually present at birth or during the first 12 months with progressive head enlargement due to megalencephaly. The disease generally takes a relatively mild course and was once known as “leukodystrophy with an unusually mild course.” Clinical manifestations are highly variable and often include epileptic seizures and ataxia due to cerebellar involvement. Despite the relatively impressive cerebral changes, deterioration of motor skills occurs late in the course of the disease, and some children are unable to walk. Usually there is only mild to moderate intellectual impairment.
MRI findings Despite the relatively mild clinical course, cerebral MRI shows very pronounced changes with diffuse white-matter swelling and the subcortical cysts that give the disease its name. The cysts become larger and more numerous as the disease progresses. T1w images show decreased signal intensity in the affected white matter; T2w images show a signal increase ( ▶ Fig. 7.7). While involvement of the subcortical U-fibers is usually seen at an early stage, only some cases manifest changes in the posterior internal capsule, thalamus, and basal ganglia. The typical cysts are best demonstrated in FLAIR images. They are often located in the frontoparietal region and anterior temporal lobe.

Fig. 7.7 Megalencephalic leukoencephalopathy with subcortical cysts (Van der Knaap’s disease) in a 22-year-old male. The patient had a known history of the disease since childhood. (a) Axial T2w image shows pronounced subcortical hyperintensities in the temporal region. (b) Axial FLAIR image shows marked, cystlike subcortical hypointensities. (c) Axial T1w image shows pronounced subcortical hypointensities in the high frontal region. Gyral swelling had been present in childhood, while current images show increased atrophy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree