Surgical resection
Stereotactic radiosurgery
Patient selection
Tissue diagnosis
Confirms the lesion is a tumor
Cannot confirm the lesion is a tumor
Lesion size
Large, ≥1.5 cma; especially if there is mass effect
Small, ≤2.0 cma; significant mass effect absent
Surgical candidate
Yes
No (or patient declines surgery)
Treatment outcome
Tumor status
Removed (≥94 % on average) [4]
Not removed
Local control
85 % for >40 months (up to 5 years) [23]
85 % at 12 months; 65 % at 24 months [25]
Local recurrence rate
Median survival time
Complications
Presentation
Usually immediate
Frequently delayed; necrosis may necessitate surgical resection
Major neurological
7 % in eloquent brain; 6 % overall [4]
25 % in eloquent brain (RTOG grade 3) [24]
Mortality (30-day)b
<2 %
1.8 %
Cost-effectiveness
Based on 3-day hospital stay
Based on 1-day outpatient stay; includes no costs of maintenance steroids, follow-up office visits, or follow-up MRI
Quality of life
Relief from mass effect
Immediate
Delayed
Steroid use
Tapered off over 2–4 weeks
Can last for months; may cause dependence
Follow-up visits
Few
Many
Patient debilitation
Not debilitated at home
Debilitated at home
Patient Selection
When a patient presents with a brain metastasis and symptoms of mass effect, there is seldom a dispute that the lesion should be removed surgically. Similarly, when a patient with a brain metastasis presents in too poor a medical condition to be a surgical candidate (or declines surgery), SRS represents a logical treatment modality. Other patients with single brain metastases can be sorted into three groups. The first group has relatively large lesions (exceeding 3 cm in maximum diameter) that can only be effectively removed by surgical resection. Treatment of these tumors with SRS is not effective because the radiation dose must be reduced as the tumor size increases, to prevent damage to surrounding brain tissue. This relationship was clearly demonstrated by Mehta et al. [25], who showed that with SRS, the rate of complete response (CR; total disappearance of the magnetic resonance [MR] image of the lesion after SRS) falls off dramatically with tumor volume such that if a tumor 2 cm3 in volume has a 50 % CR rate, an 8–9-cm3 lesion shows about a 20 % CR rate (Fig. 15.1).
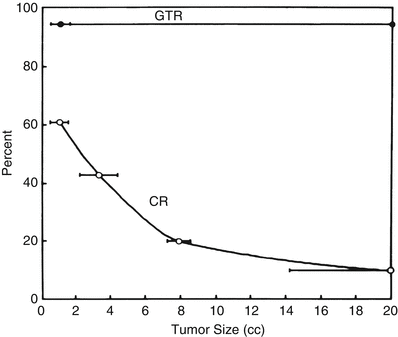

Fig. 15.1
Tumor size versus response to radiosurgery and surgery for brain metastases. Comparison of a representative radiosurgery complete response (CR) curve [25] with a surgery gross-total resection (GTR) curve (average percentage, 94 % from Table 15.2), showing that CR rate with radiosurgery is tumor size dependent, whereas GTR rate with surgery is not (From Sawaya R. Surgical treatment of brain metastases. Clin Neurosurg 1999; 45:41–47. Used with permission)
Patients in the second group have very small lesions (less than 10 mm in maximum diameter) that are not surgically accessible and are located deep within the brain. In this situation, SRS provides an effective alternative to resection, superior to WBRT, which was the only treatment previously available.
The third group of patients are those who have metastases that are less than 3 cm in maximum diameter and are surgically accessible. Whether surgery or SRS is the best treatment for these lesions is the subject of much current debate.
The 3-cm upper size limit referred to above is probably too high for adequate SRS treatment. At M.D. Anderson, a relatively recent study of 153 brain metastases from melanoma treated with SRS [28] showed that the 1-year local control rate of smaller tumors with a maximum diameter of no more than 1.5 cm (volume = 2 cm3) was superior to that of larger lesions (75.2 % and 42.3 %, respectively; p < 0.05). Moreover, another study at M.D. Anderson used SRS to treat brain metastases from different primary tumor types [26] and found a 1-year actuarial local control rate of 86 % for lesions that were 1 cm or less in maximum diameter (0.5 cm3) but only a 56 % local control rate for lesions larger than this (p = 0.0016). Furthermore, an earlier study by a radiosurgery group in Pittsburgh [29] included only brain metastases that were no larger than 2.5 cm in maximum diameter and found that treatment with SRS plus WBRT was superior to WBRT alone. It is important to note that control rates similar to surgery for lesions as large as 2 cm (volume ~5 cm3) can be achieved with the addition of fractionated WBRT [30]. Collectively, these findings clearly indicate that for effective use of SRS alone (i.e., without the addition of WBRT), a cutoff point in brain metastasis diameter that is lower than 3 cm is warranted.
Treatment Outcome: Survival
The controversy remains as to whether surgery or SRS is more appropriate and effective to treat patients in the group with single brain metastases that are surgically accessible and range between 1 and 3 cm in maximum diameter. Because no one has yet been able to complete a prospective randomized study comparing treatment of such patients with surgical resection and SRS, the question of which of the two is more effective lacks a definitive answer.
In lieu of this, many retrospective studies have been performed comparing surgical resection and SRS for brain metastasis treatment. Among the more carefully constructed studies of this type are those of Auchter et al. [16], Bindal et al. [23], and Cho et al. [31].
Auchter and coworkers [16] performed a multi-institutional retrospective outcome and prognostic factor analysis of patients with single cerebral metastases who were treated with SRS plus WBRT. From their database of 533 patients with brain metastases treated with SRS and WBRT, they selected 122 patients who fulfilled the criteria for surgical resection established in the prospective randomized trial of Patchell et al. [13], including having a single brain metastasis that was surgically resectable (but no urgent need for surgery), no prior radiotherapy or surgical treatment, independent functional status (a Karnofsky Performance Scale [KPS] score >70), and a non-radiosensitive tumor. They then compared the outcome of these 122 patients with that of the patients in the prospective randomized surgical series of Patchell et al. [13] and Noordijk et al. [12]. The actuarial median survival time after treatment with SRS plus WBRT was 56 weeks compared with 40 weeks [13] and 43 weeks [12], respectively, after surgery plus WBRT. The median duration of functional independence was 44 weeks after SRS and WBRT [16] compared with 38 weeks [13] and 33 weeks [12], respectively, after surgery and WBRT. These results indicated to Auchter et al. [16] that SRS plus WBRT produced outcomes for patients with single brain metastases that were comparable with, if not better than, surgery plus WBRT.
Cho and coworkers [31] retrospectively reviewed a series of 225 single brain metastases in patients treated with either WBRT alone, SRS plus WBRT, or surgery and WBRT. There was a similar distribution of prognostic factors such as age, sex, KPS score, and metastasis location in these three groups except for extracranial cancer, which was 14 % higher in the group treated with SRS and WBRT. The actuarial median survival times for patients treated with SRS and WBRT (9.8 months) and surgery plus WBRT (10.5 months) were nearly identical, but both were significantly better than for those treated with WBRT alone (3.8 months). Other more recent retrospective studies have also labeled resection and SRS as equally effective in the treatment of small to moderately sized brain metastases [32].
At M.D. Anderson, Bindal and coworkers [23] also retrospectively compared surgical resection and SRS treatment of brain metastases. Thirty-one patients treated with SRS were followed prospectively, and 62 patients treated with conventional surgery were retrospectively matched to those in the SRS group. Patients were matched according to primary tumor histology, extent of systemic disease, preoperative KPS score, time to brain metastasis, number of brain metastases, and patient age and sex. WBRT treatment was similar in both groups, and patient eligibility criteria for SRS were the same as for surgery. Lesions treated by SRS were limited to those <3 cm in maximum diameter, and 81 % of them were deemed surgically resectable. There was a statistically significant survival advantage in the surgically treated group (16.4 months median survival) relative to the group treated with SRS (7.5 months median survival) (p = 0.0009 by multivariate analysis). In contrast to the conclusions of Auchter et al. [16] and Cho et al. [31], Bindal’s group [23] concluded that surgery was superior to SRS in clinically similar patients in terms of survival, local recurrence, and morbidity. Thus, determination of whether treatment of brain metastases with SRS is better than, equivalent to, or worse than surgical resection must await a phase III prospective trial comparing SRS with conventional surgery for the treatment of single brain metastases.
Tumor Local Control and Recurrence
When brain metastases are removed surgically, local control is produced by total removal of the tumor and elimination of surrounding edema (Fig. 15.2). At M.D. Anderson, gross-total resection (equivalent to complete response in radiosurgery) of metastatic tumors is achieved in 90–96 % of instances, in both eloquent and noneloquent brain regions (Table 15.2). With surgery, local control (absence of recurrent tumor growth after gross-total resection) typically persists at an 85 % level at 1 year and remains there for an interval ranging from 40 months to 5 years (Fig. 15.3).
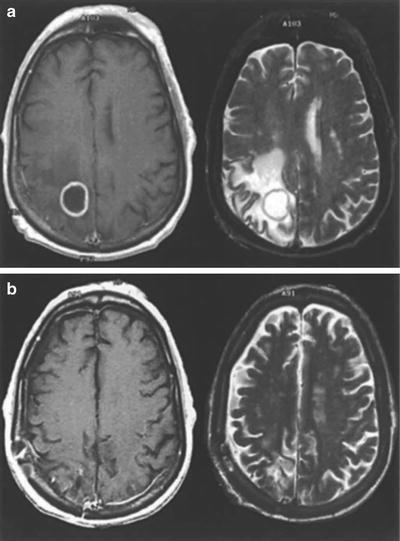
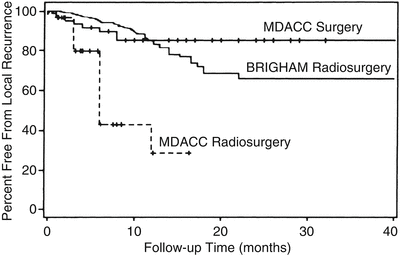

Fig. 15.2
(a) Preoperative and (b) postoperative gadolinium contrast-enhanced MR images of a brain metastasis in a patient with non-small cell lung cancer. In each panel, the left and right images are T1- and T2-weighted, respectively (From Sawaya R. Surgical treatment of brain metastases. Clin Neurosurg 1999; 45:41–47. Used with permission)
Table 15.2
Gross-total resections performed for metastatic tumors in different brain regions (gross-total resection is equivalent to a complete response in radiosurgery)
Metastasis location (tumor functional gradea) | No. of patients | GTR (%) |
|---|---|---|
Noneloquent (I) | 79 | 75 (95) |
Near-eloquent (II) | 61 | 55 (90) |
Eloquent (III) | 54 | 52 (96) |
Total | 194 | Average, 94 |

Fig. 15.3
Freedom from local recurrence (local control) of brain metastases with radiosurgery and with surgery. Curve showing time from radiosurgical treatment to local failure (BRIGHAM Radiosurgery) in 42 patients treated at Brigham and Women’s hospital [25] superimposed on curves showing time from surgical resection (MDACC Surgery) to local recurrence in 62 patients and time to local failure in 31 patients radiosurgically treated (MDACC Radiosurgery) at The University of Texas M.D. Anderson Cancer Center [23] (From Sawaya R. Surgical treatment of brain metastases. Clin Neurosurg 1999; 45: 41–47. Used with permission)
In contrast, when brain metastases are treated with SRS, it is common for the tumor to remain visible on computed tomography (CT) or MR images. Local tumor control with SRS is less stringently defined and, in addition to tumor regression, includes lesions remaining unchanged in size as well as those undergoing no more than a 25 % increase in size/volume above the baseline measurement. This makes it difficult and misleading to compare values published for local control of brain metastases by surgical resection and SRS. In some patients who have a brain metastasis that is stable on MR images or increases in size by less than 25 %, surgery may be required to relieve neurologic deficits resulting from mass effect and persistent edema (Fig. 15.4) [33].
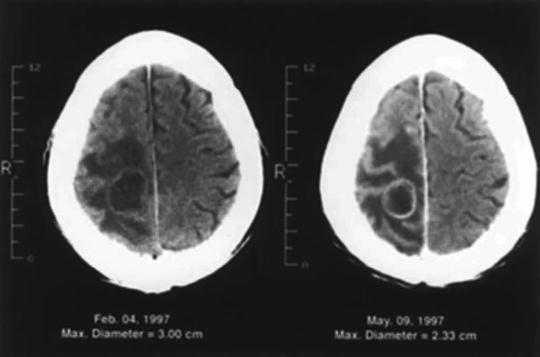

Fig. 15.4
CT scan of a patient with non-small cell lung cancer who had a 3-cm brain metastasis in the right anterior parietal lobe (left) that was treated with radiosurgery. Three months later, the tumor shrank by 23 %, but the edema and the patient’s symptoms were unchanged (right). The patient subsequently underwent a craniotomy and total resection of the mass. The results of the surgery are shown in Fig. 15.2 (From Sawaya R. Surgical treatment of brain metastases. Clin Neurosurg 1999; 45:41–47. Used with permission)
After surgical resection of brain metastases at M.D. Anderson, we have observed local tumor recurrence at a rate ranging from about 8–12 % [4, 23], which is consistent with the observations of others. In patients treated for brain metastases with SRS, similar 1-year local control failure rates (6–15 %) are often reported [16, 18, 19, 34–38]. These numbers reported for SRS are flawed not only because of the less stringent definition of local control with SRS but also because tumor recurrence in brain metastasis patients is a function of survival time, and many radiosurgical series of these patients report such short survival intervals (8 months on average) that failure of local tumor control cannot be reliably determined. Treatment of brain metastasis patients with SRS at Brigham and Women’s Hospital [25] gave an 85 % local control rate at 1 year, but this dropped steadily to 65 % after 2 years (Fig. 15.3). More recent series of brain metastasis patients treated with SRS have presented overall recurrence values (30–47 %) [26, 27] that are much higher than those typical of surgically treated patients. These numbers may be more realistic, reflecting a trend toward increased follow-up monitoring of patients, with more routine neuroimaging [28].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree