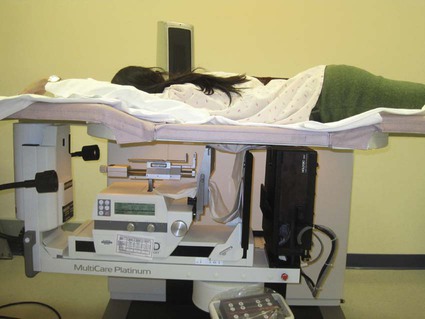
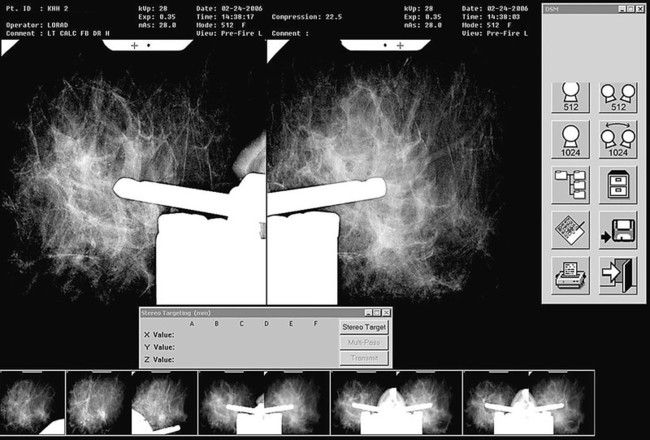
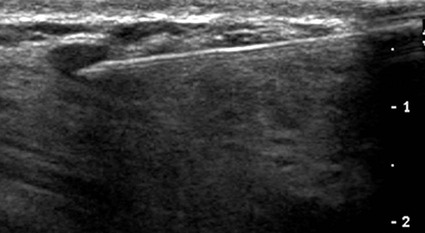
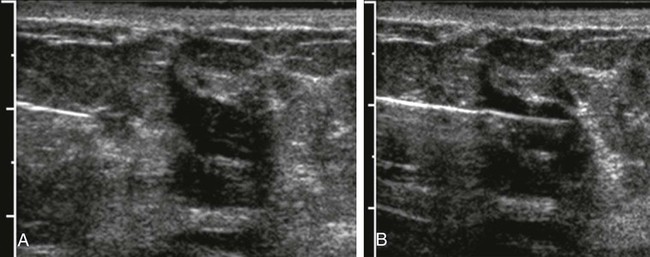
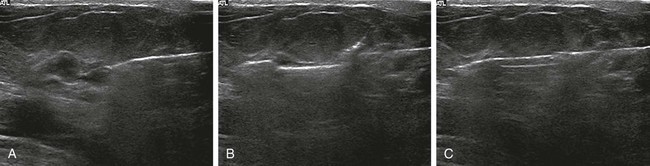
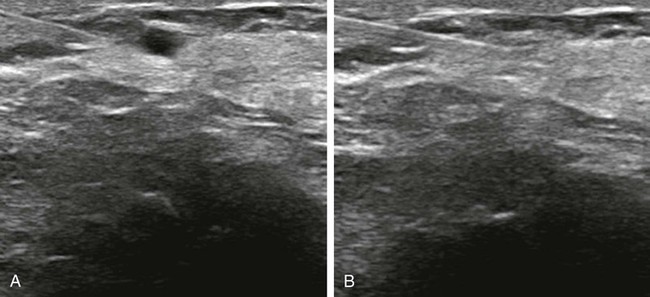
Screening mammography has been shown to decrease the incidence of mortality associated with breast cancer.1 Since the initial implementation of widespread screening mammography programs, breast imaging has evolved to include full-field digital mammography (FFDM), ultrasound (US), magnetic resonance imaging (MRI), and more recently, molecular imaging techniques including breast-specific gamma imaging (BSGI) and positron emission mammography (PEM) to aid in the evaluation of breast pathology. The ultimate goal of these technologic advances is to allow for the detection of small and early-stage breast cancer, thus providing patients with the best chance of cure and the least amount of treatment-associated morbidity. However, with the detection of smaller lesions comes the challenge of developing methods to arrive at a pathologic diagnosis in an accurate, cost-effective, and safe manner. Wire-guided surgical breast biopsy was the gold standard for the diagnosis of nonpalpable radiographically detected breast lesions, but given that 80% of indeterminate breast lesions that undergo biopsy are subsequently found to be benign, development of less invasive, more cost-effective techniques was needed.2 Targeting mammographically detected lesions for minimally invasive biopsy employs stereotactic guidance. The most common type of suspicious mammographic abnormality targeted for biopsy via this method are microcalcifications, although any suspicious abnormality seen solely or best with mammography, including masses or asymmetries, can be targeted and biopsied via stereotaxis. In a large multicenter trial performed in 2003, investigators found that 70% of stereotactic vacuum-assisted biopsy (VAB) was performed for lesions containing suspicious microcalcifications.3 For a stereotactic breast biopsy, either a dedicated table is needed or an attachment to a digital mammography unit and a special chair to allow for proper patient positioning. If a dedicated table is used, the patient is prone with the breast positioned dependently through an aperture in the table (Fig. 155-1). Attached to the underside of the table is a mammography unit on an articulating arm, which can be moved such that +15-degree and −15-degree images are obtained. These angles are used by the computer to determine the coordinates of the targeted lesions to within 1 mm of accuracy. If a standard mammography unit with an attachment allowing stereotactic guidance is used, a chair can be positioned to optimally target the lesion. The advantages of a dedicated stereotactic table include greater comfort for the patient and physician and no risk of a vasovagal reaction. However, it requires a dedicated room and space. A mammography unit allows for more efficient use of space and equipment because it can be used for routine imaging when not being used for biopsy. This latter solution may be best suited for practices with lower volumes where use of a mammography unit for biopsy will not impact workflow of screening and diagnostic mammography. The overlying skin is prepped in a sterile fashion, followed by injection of topical anesthetic into the skin and subcutaneous and parenchymal tissue.4 A 3-mm incision is made with a scalpel, and the biopsy probe is advanced through this incision to the predetermined depth. Typically, 6 to 12 samples are obtained. Following biopsy, the device is removed, and a titanium marker is placed through the biopsy probe to denote the area of biopsy. A postprocedure mammogram is critical to confirm the targeted lesion was indeed biopsied, determine clip placement, and have a new baseline; an image of the entire breast rather than the small field of view of the stereotactic device is paramount (Fig. 155-2). US is an indispensable tool in the breast imager’s armamentarium. This technology not only allows for differentiation of cystic versus solid lesions but can also provide more detailed characterization of solid lesions into probably benign, indeterminate, or suspicious categories.5–14 This detailed evaluation has largely been made possible by the introduction of high-frequency 7.5- to 10-MHz linear array transducers in the mid-1990s. The ability to carefully evaluate a mass sonographically has proven extremely useful clinically and can improve the specificity of mammographically detected abnormalities. Findings at sonography can also serve to help tailor interventional procedures. US allows for accurate diagnosis of a mammographic or palpable mass as a simple cyst, a benign lesion. Specific criteria have been developed to allow for classification of a breast lesion as a cyst. When all criteria for a simple cyst are strictly adhered to, the accuracy of US is 96% to 100%.15 These criteria are demonstration of an imperceptible wall, through transmission of sound, and lack of internal echoes (anechoic). Cysts are typically round or oval in shape and can vary in size from several millimeters to several centimeters. Once a lesion has been diagnosed as a simple cyst, intervention may occur for one of several reasons. Most commonly, the patient may be complaining of a palpable lump or pain and tenderness related to the cyst, and aspiration may alleviate these symptoms.16 However, it is important to relate to the patient that recurrence of a cyst after aspiration is very common. A second reason to aspirate a benign cyst is very large size, which may obscure a significant portion of the breast and thereby limit mammographic interpretation. In this case, the cyst is aspirated and the mammogram is repeated to allow adequate evaluation. A cyst is typically aspirated under sonographic guidance (Fig. 155-3). The lesion is first localized, and a skin entry site is chosen. The skin is then prepped with alcohol, and intradermal and subcutaneous local anesthesia is injected. Under direct sonographic visualization, a 22- to 25-gauge needle is advanced into the lesion, and the fluid contained within the cyst is aspirated through sterile tubing into a syringe. At many facilities, the aspirated fluid may be discarded if it is clear, straw-colored, green, milky or “motor-oil” colored, since these are compatible with benign cystic fluid. If the cyst contains bloody fluid, even if the sonographic criteria of a simple cyst were met, the fluid should be sent for cytologic evaluation. Through the years, the stereotactic biopsy needle has undergone many developments. Initial stereotactic biopsy efforts were focused on FNA or biopsy with a spring loaded-device, which required removal from the breast following every sample. However, high insufficiency rates (10%-38%)17,18 plus the inability to determine invasion from ductal carcinoma in situ (DCIS) were significant limitations to FNA, and substantial undersampling, particularly of calcifications with a spring-loaded device, resulted in important modifications to this original technique. Gradually the gauge of biopsy needles increased to the now used 11-, 9-, 8-, and even 7-gauge probes. Further development followed the introduction of VAB to actively draw tissue into the biopsy chamber, as opposed to sampling that tissue which was passively in the specimen chamber. This resulted in larger tissue samples with decreased sampling error and improved accuracy of diagnosis.19–24 Because of the potential for calcifications to be histologically heterogeneous (e.g., regions of both atypical ductal hyperplasia [ADH] and DCIS), biopsy performed with a larger-gauge needle, along with directional vacuum assistance, has proved very helpful in obtaining larger samples and decreasing underestimation rates.25–28 Overall, in comparison to CNB, underestimation drops about 1.8-fold when using vacuum-assisted devices.29 Therefore, directional VAB is now used to biopsy lesions with mammographically detected microcalcifications. An additional advantage of the use of a VAB probe is that it does not have to be removed and reinserted. Instead, the vacuum draws the tissue into the specimen chamber, cuts it, and transports the specimen into a collection chamber at the other end of the device. The result is that multiple samples can be obtained while the needle remains in the breast. The vacuum device has been shown to produce tissue samples larger in size and heavier in weight.29–32 Acquisition of larger tissue samples significantly increases the accuracy of diagnosis of ADH and DCIS.33 The reported rates of upgrade for ADH lesions average 44% for 14-gauge spring-loaded stereotactic core biopsy, 24% for 14-gauge directional vacuum-assisted breast biopsy, and 19% for 11-gauge vacuum-assisted breast biopsy needles (range, 0%-58%). The diagnosis of ADH following percutaneous biopsy is an indication for surgical excision to exclude the diagnosis of breast carcinoma. A diagnosis of lobular atypia at minimally invasive biopsy remains controversial, with some institutions not recommending surgical excision at all. Increasingly, the recommendations are for surgical excision of all lobular neoplasia diagnosed at minimally invasive biopsy, or the excision of pleomorphic lobular carcinoma in situ.34,35 FNA may be performed on cystic, solid, or mixed solid lesions. A 22- to 25-gauge needle is used, and the technique is similar to the aforementioned description of cyst aspiration. Typically, several passes are made with the needle through different parts of the lesion with a back and forth motion while aspirating with a syringe (Fig. 155-4). Benefits of FNA include decreased cost of procedure and decreased likelihood of hematoma. However, as stated earlier, there are significant and well-established limitations to FNA. The success of FNA is operator dependent, with the best results achieved when performed by an experienced operator. Insufficiency rates have been found to improve with the presence of a cytopathologist during the procedure.36 Another disadvantage of FNA is its inability to differentiate between DCIS and invasive cancer when malignant ductal cells are identified due to the lack of tissue architecture. Therefore, lesions chosen for biopsy with FNA should be selected based on the sonographic appearance, with less suspicious lesions favored for biopsy with this method, to lessen the likelihood of re-biopsy with a larger gauge needle to establish a diagnosis, or in clinical situations where a core biopsy is more challenging, such as when a lesion is immediately abutting an implant or large vessel. Radiologic-pathologic concordance is paramount when performing FNA. US-guided CNB has been shown to be a reliable alternative to surgical excision.37,38 The procedure is cost-effective and well tolerated by patients and has become a mainstay in clinical practice. US-guided CNB enables accurate diagnosis of malignancy and allows for improved surgical planning for treatment. Even lesions that are considered highly suspicious for malignancy should undergo biopsy prior to definitive surgical procedure; this will alleviate the need for a second surgical procedure for nodal sampling. Estrogen and progesterone receptor status, as well as Her-2 status, can be ascertained on a CNB and are generally performed on the biopsy obtained at minimally invasive biopsy. Such assessment can assist with patient counseling and treatment options prior to surgery and can result in a less traumatic and more complete discussion of surgical options. Studies have shown that when a diagnosis of cancer is known prior to lumpectomy/partial mastectomy, the likelihood of negative margins is higher as the surgical excision is more extensive.39 Additionally, it can reduce the need for repeat surgeries to evaluate and/or treat the axilla in the case of a diagnosis of invasive versus in situ disease. These procedures can be performed utilizing automated or vacuum-assisted devices. Automated CNB devices are typically spring loaded. Once the skin has been prepped and anesthetized and the CNB device has been advanced under US guidance, it should be placed 1 to 3 cm proximal to the edge of the lesion. The device is fired into the lesion, resulting in a core biopsy specimen that maintains the architecture of the lesion, thereby allowing for a surgical-quality specimen (Fig. 155-5). Real-time documentation of the needle within the lesion is critical to confirm sampling of the targeted lesion. The “throw” of the needle varies with the manufacturer, as does the gauge of the needle and chamber size. It has been noted in the literature that one disadvantage of this US-guided CNB using a spring-loaded device is the need for multiple reinsertions of the needle through the skin incision. Typically, three to five passes are made through various aspects of the lesion, thereby sampling all quadrants of the lesion and decreasing sampling error. There has been debate in the literature as to the number of specimens needed to achieve a reliable histologic diagnosis, with some groups reporting the greatest accuracy achieved with three passes,40 and over 90% accuracy in the first pass.41 A more recent development for US-guided percutaneous breast biopsy is the availability of VAB probes. These are larger-gauge needles9,11 and allow for procurement of larger cores under real-time imaging (Fig. 155-6). These devices obtain a larger amount of tissue to further increase accuracy of diagnosis, similar to stereotactic-guided vacuum-assisted devices. Additionally, these are typically single-insertion devices, eliminating the need for multiple reentries into the breast. These VAB probes may be useful when a lesion is sonographically visible but also contains microcalcifications, and therefore inherently contain the issues of heterogeneity and underestimation of disease associated with microcalcifications, as previously discussed. The use of sonographically guided VAB is increasingly being used to excise biopsy-proven benign masses, such as fibroadenomas, when excision is desired but surgery is not. This requires additional time, but with the acquisition of sequential samples, the sonographically visible lesion can be excised. Of course, this does not ensure complete pathologic excision. Most lesions biopsied under sonographic guidance are masses.42 If the mass is sufficiently small that there is concern that the entire lesion is removed, or if there is a possibility of neoadjuvant chemotherapy with subsequent disappearance of the lesion, a metallic marker should be placed immediately following biopsy to identify the lesion’s precise location. In our practice, all lesions that undergo biopsy have a metallic clip placed to ensure accurate localization of the lesion that underwent biopsy. Most masses are adequately sampled with a spring-loaded device, usually 12 to 14 gauge. However, in certain clinical situations, such as with intraluminal lesions where there is the possibility of a papillary lesion, use of sonographically guided VAB may be helpful. In the specific case of papillomas, it was found in a recent study that the sensitivity and specificity at automated CNB was 28% and 100%, respectively. For vacuum-assisted CNB, both sensitivity and specificity were found to be 100%.43 Therefore, use of sonographically guided VAB if the diagnosis of a papilloma is favored may be preferential. Confirmation of lesion retrieval is an essential component of breast biopsy. For nonpalpable breast lesions, whether biopsied via percutaneous or open surgical technique, confirmation of lesion retrieval is achieved by specimen radiography, postbiopsy mammograms, and by histologic-radiographic correlation. If calcifications are targeted, then calcifications must be present in the specimen radiograph, with morphologic features corresponding to the calcifications originally targeted. It is sometimes helpful to place the specimens that contain the calcifications separately so pathologists can identify them easily and examine them preferentially. Histologic-imaging discordance (i.e., where the pathologic diagnosis does not explain the imaging finding) can signal failure of lesion retrieval and may be an indicator of the need for either re-biopsy or surgical excision. Examples of histologic-imaging discordance include: (1) calcified lesions failing to demonstrate calcification on specimen radiography, histology, or both, (2) lesions suspicious for malignancy where histology does not account for imaging findings (i.e., a mass where the pathology demonstrated only fibroadipose tissue, which does not explain the radiographic presence of a mass), and (3) lesions highly suspicious for malignancy that yield benign histology, such as Breast Imaging Reporting and Data System (BIRADS) 5 lesions. The frequency of discordance reported in the literature varies from 0.9% to 6.2%.44 When evaluating specimen radiographs of the sample, calcification retrieval rates have been reported ranging from 95% to 100% of cases.3,45–47
Minimally Invasive Image-Guided Breast Biopsy and Ablation
Introduction
Stereotactic Interventions
Ultrasound-Guided Interventions
Biopsy Devices
Stereotactic Biopsy
Ultrasound-Guided Biopsy
Lesion Retrieval and Radiologic-Pathologic Concordance
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine