Morbidly adherent placenta (MAP) encompasses a spectrum of conditions characterized by abnormal adherence of the placenta to the implantation site. Classification of MAP is based on the degree of trophoblastic invasion through myometrium and uterine serosa and includes accrete, when the villi are attached to the myometrium but do not invade the muscle; increta, when the placenta invades partially through the myometrium; and percreta, when it invades up to and beyond the uterine serosa. Knowledge of the common findings of MAP on MR imaging is important to be able to provide an accurate diagnosis.
Key points
- •
Incidence of morbidly adherent placenta (MAP) is increasing with the incidence of cesarean sections.
- •
Ultrasound is the primary screening technique for evaluation of MAP, and MR imaging is used if ultrasound is limited or equivocal for high-risk patients and for delivery planning.
- •
MR imaging should be performed between 24 and 32 weeks of gestational age to avoid common pitfalls.
Introduction
Morbidly adherent placenta (MAP) encompasses a spectrum of conditions characterized by abnormal adherence of the placenta to the implantation site. Classification of MAP is based on the degree of trophoblastic invasion through myometrium and uterine serosa and includes accreta, when the villi are attached to the myometrium but do not invade the muscle; increta, when the placenta invades partially through the myometrium; and percreta, when it invades up to and beyond the uterine serosa.
Clinically, MAP becomes a problem during delivery when the placenta does not completely separate from the uterus, which can be followed by a cascade of events, including massive hemorrhage leading to disseminated intravascular coagulation, the need for hysterectomy, surgical injury to the bladder, uterus, bowel, or neurovascular structures, adult respiratory distress syndrome, acute transfusion reaction, electrolyte imbalance, and renal failure. MAP is associated with up to one-half of emergency peripartum hysterectomies.
The incidence of MAP is increasing and seems to parallel the increase in cesarean delivery rate. The frequency of MAP has increased by more than 10-fold in the past 30 years to approximately 3 cases per 1000 deliveries. Other risk factors for MAP include any myometrial damage from previous myomectomy, endometrial defects due to vigorous curettage resulting in Asherman syndrome, submucous leiomyomas, thermal ablation, and uterine artery embolization. Placenta previa is also a risk factor for MAP; however, this may be due to the increased incidence of placenta previa in patients with prior cesarean sections. Silver and colleagues found that in the presence of placenta previa the risk of MAP was 3%, 11%, 40%, 61%, and 67% for the first, second, third, fourth, and fifth or greater repeat cesarean deliveries, respectively. This is hypothesized to be due to the increased risk of incomplete reapproximation of the incised edges as the incidence of repeat cesarean sections increases. Garmi and colleagues were able to show in vitro that an induced sharp decidual incision, which would imitate the in vivo process of a cesarean section, increased the invasion potential of trophoblastic cells. Placenta previa without prior cesarean delivery confers a very low risk of MAP, of about 1% to 5%.
Maternal mortality with MAP has been reported to be as high as 7%. The average blood loss at delivery in women with MAP is estimated to be 3 to 5 L ; as many as 90% of patients with MAP require blood transfusion, with 40% requiring more than 10 units of packed red blood cells. A multidisciplinary team including individuals with special expertise in high-risk obstetrics, gynecologic surgery, urology, radiology, obstetric anesthesia, and blood banking has been shown to reduce mortality.
Introduction
Morbidly adherent placenta (MAP) encompasses a spectrum of conditions characterized by abnormal adherence of the placenta to the implantation site. Classification of MAP is based on the degree of trophoblastic invasion through myometrium and uterine serosa and includes accreta, when the villi are attached to the myometrium but do not invade the muscle; increta, when the placenta invades partially through the myometrium; and percreta, when it invades up to and beyond the uterine serosa.
Clinically, MAP becomes a problem during delivery when the placenta does not completely separate from the uterus, which can be followed by a cascade of events, including massive hemorrhage leading to disseminated intravascular coagulation, the need for hysterectomy, surgical injury to the bladder, uterus, bowel, or neurovascular structures, adult respiratory distress syndrome, acute transfusion reaction, electrolyte imbalance, and renal failure. MAP is associated with up to one-half of emergency peripartum hysterectomies.
The incidence of MAP is increasing and seems to parallel the increase in cesarean delivery rate. The frequency of MAP has increased by more than 10-fold in the past 30 years to approximately 3 cases per 1000 deliveries. Other risk factors for MAP include any myometrial damage from previous myomectomy, endometrial defects due to vigorous curettage resulting in Asherman syndrome, submucous leiomyomas, thermal ablation, and uterine artery embolization. Placenta previa is also a risk factor for MAP; however, this may be due to the increased incidence of placenta previa in patients with prior cesarean sections. Silver and colleagues found that in the presence of placenta previa the risk of MAP was 3%, 11%, 40%, 61%, and 67% for the first, second, third, fourth, and fifth or greater repeat cesarean deliveries, respectively. This is hypothesized to be due to the increased risk of incomplete reapproximation of the incised edges as the incidence of repeat cesarean sections increases. Garmi and colleagues were able to show in vitro that an induced sharp decidual incision, which would imitate the in vivo process of a cesarean section, increased the invasion potential of trophoblastic cells. Placenta previa without prior cesarean delivery confers a very low risk of MAP, of about 1% to 5%.
Maternal mortality with MAP has been reported to be as high as 7%. The average blood loss at delivery in women with MAP is estimated to be 3 to 5 L ; as many as 90% of patients with MAP require blood transfusion, with 40% requiring more than 10 units of packed red blood cells. A multidisciplinary team including individuals with special expertise in high-risk obstetrics, gynecologic surgery, urology, radiology, obstetric anesthesia, and blood banking has been shown to reduce mortality.
Why MR imaging?
Ultrasound (US) is the primary diagnostic tool for MAP and is performed as the initial screening examination. In patients with a placenta previa or a low-lying placenta after the initial screening examination performed at 18 to 20 weeks, a repeat examination at 32 weeks is recommended to evaluate the placental location. US has been reported to have a sensitivity of 91% and a specificity of 97% for the diagnosis of MAP in a recent meta-analysis and is able to evaluate for MAP in most cases. MR imaging is indicated when the US evaluation is limited or equivocal for patients with high clinical risk factors for MAP. In cases where US has provided a definitive diagnosis, MR imaging is usually performed for delivery planning because it is able to outline the anatomy of the invasion, relate it to regional vascular system, and confirm parametrial invasion and possible ureteral involvement.
How to do it?
MR imaging sequences with high temporal resolution and good contrast-to-noise ratios are used for placental imaging. MR imaging is typically performed on a 1.5-T system; however, recently 3 T has been used by some institutions to perform their fetal imaging as well. A study by Victoria and colleagues comparing 1.5 T and 3 T for fetal imaging found that although some structures like spine and cartilage were better seen on 3 T and their imaging scores improved with increasing gestational age, overall, more imaging artifacts were seen on the 3-T MR imaging. No specific articles exist to date evaluating the usefulness of 3-T MR imaging in placental imaging. The American College of Radiology (ACR)–Society for Pediatric Radiology Practice Parameter for safe and optimal performance of fetal MR imaging suggests absence of any reported adverse effects to the mother or developing fetus at 3 T or weaker magnetic fields; however, the investigators acknowledge that most of their data were from research involving 1.5 T or less. A multichannel phased array coil is typically used, and the examination typically takes about 15 to 30 minutes to complete. If the patient cannot tolerate a supine position, especially in the later part of pregnancy, a left lateral decubitus or oblique position may be better tolerated. This position decreases the risk of impaired venous return from caval compression by the uterus. Ideally, the bladder is only partially distended both for patient comfort and to be able to evaluate bladder wall invasion if present. Breath-hold techniques can be used to minimize breathing motion artifacts.
Single-shot T2-weighted (T2W) echo-planar fast spin-echo sequences in multiple planes are used to image the placenta. Acquisitions in axial, sagittal, and coronal plane with respect to the uterus are performed to image all regions of the placenta. True fast imaging with steady state precession (FISP) can also be used to help eliminate artifacts caused by maternal and fetal motion. At the author’s institution, they acquire True FISP images in all 3 planes as well. T1-weighted images can be acquired to evaluate for areas of hemorrhage if placental abruption is of concern; however, T1-weighted images need not be performed as routine. Gadolinium-based contrast agents (GBCA) can add to the diagnostic value of T2-weighted imaging (T2WI) for the diagnosis of placenta accreta; however, they are rarely used in pregnant women due to persistent uncertainty regarding fetal risks posed by GBCA crossing the placenta. Millischer and colleagues found an increase in the accuracy of diagnosis in both the senior and the junior radiologist on addition of gadolinium to their examinations. The Contrast Media Safety Committee of the European Society of Urogenital Radiology reviewed the literature and determined that no effect on the fetus has been reported following the use of gadolinium contrast media ; however, the ACR guidelines recommend that intravenous gadolinium should be avoided during pregnancy and should be only used if absolutely essential. The protocol used at the author’s institution is shown in Table 1 . In addition, a radiologist is always available to review the images before the patient is taken off the gantry table and to suggest any additional or oblique images if necessary.
| Sequence | Planes | TR (ms) | TE (ms) | FOV (cm) | Slice Thickness (mm) | Matrix | ETL | Flip Angle (°) |
|---|---|---|---|---|---|---|---|---|
| Ultra-fast spin-echo T2 half-Fourier | Axial, coronal sagittal | 1800 | 64 | 32–40 | 5 | 256 × 160 | 256 | 160 |
| True FISP/BFFE | Axial, coronal, sagittal | 411 | 1.8 | 32–40 | 5 | 256 × 182 | 1 | 62 |
| T1-weighted TSE | Axial | 684 | 10 | 32–40 | 6 | 320 × 240 | 3 | 180 |
| T2-weighted TSE | Sagittal | 4650 | 79 | 32–40 | 5 | 256 × 256 | 19 | 144 |
| DWI (b values = 0, 400, 800 s/mm 2 ) | Axial | 6800 | 60 | 38 | 7 | 140 × 140 | 1 | 90 |
Recently, the utility of diffusion-weighted imaging (DWI) in defining placental invasion was evaluated by Morita and colleagues. DWI at a b value of 1000 s/mm 2 clearly defined the border between the placenta and myometrium because only the placenta showed very high signal intensity at this high b value. The corresponding image at a b value of 0 s/mm 2 showed the myometrium with high signal intensity compared with the surrounding fat. They postulate that the fusion of these 2 images would be able to provide better diagnosis of placenta accreta.
When to do it?
Horowitz and colleagues found that placental MR imaging examinations performed before 24 weeks showed an unacceptable accuracy, sensitivity, positive predictive value, and likelihood ratio for the detection of abnormal placentation. They recommend waiting to perform the placental MR imaging after 24 weeks even if the 20-week screening US is suspicious for abnormal placentation. Placental MR imaging examinations performed at or after 24 weeks in their study had a very high positive predictive value of 96%, good sensitivity of 79%, and specificity of 94%, which was better than those examinations performed before 24 weeks. D’Antonio and colleagues suggest that serial follow-up scans in the third trimester, starting from 28 weeks of gestation, are needed in order to predict accurately the extent of the invaded area and to plan the best surgical treatment.
Imaging features
Several signs have been described on MR imaging to help diagnose MAP or placenta accreta, with some of the common ones listed in Box 1 .
Placenta previa
Uterine bulging
Heterogenous signal intensity within the placenta
Dark intraplacental bands on T2-weighted images
Focal interruptions in the myometrial wall
Direct visualization of the invasion of pelvic structures by placental tissue
Normal Myometrium and Placenta
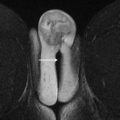
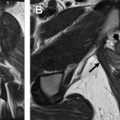
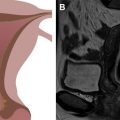
On T2WI, placenta appears predominantly homogenous with intermediate signal intensity in the second trimester with increasing heterogeneity seen in the later gestational ages and is usually clearly distinct from the underlying myometrium ( Fig. 1 ). Regularly spaced, uniformly thin, linear areas of decreased signal intensity are seen in the myometrium, likely due to the areas of normal placental septal attachment ( Fig. 2 ). These are more commonly seen when imaged with a 3-T system and are distinct from the thicker septae seen with MAP. Normal retroplacental vascularity is seen as a thin layer of numerous flow voids just under the placenta. Myometrial thickness varies with the gestational age with progressive thinning seen later in pregnancy. The gravid uterus is pear shaped with the fundus and body being wider than the lower uterine segment (LUS), and the contour is smooth with no areas of focal bulging seen ( Fig. 3 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree