This article discusses neonatal magnetic resonance (MR) imaging and reviews equipment and procedures for MR-related transport, sedation, monitoring, and scanning. MR is gaining importance in the diagnosis and clinical management of critically ill, and often very low birth weight infants, so research is ongoing to make transport and examination safer and imaging more successful. Efforts are focused on integration of dedicated neonate MR scanners in neonatal intensive care units, improvements in incubator technology and handling, and more efficient use of scan/sedation time by choosing dedicated neonate coil arrays that improve the signal-to-noise-ratio and facilitate the choice of modern imaging techniques.
The demand for magnetic resonance (MR) imaging of severely compromised term and preterm infants in neonatal intensive care units (NICUs) is increasing worldwide because MR imaging, with its excellent image quality, has unequaled diagnostic and prognostic value. MR imaging is indicated for neonates with brain injuries and neurologic disorders. In these cases, MR imaging detects more abnormalities, and shows their position and extent more precisely, than cranial ultrasound, the safe first-line imaging modality readily available in neonatal nurseries. A recent retrospective study on 129 neonatal MR imaging examinations found that the initial ultrasound diagnosis was changed or further specified in more than 57% of patients, and MR-based changes in clinical management were initiated in 58% of patients. Recent advances in MR imaging techniques add to the demand for MR imaging, because it concurrently provides quantitative structural, functional (ie, perfusion and diffusion), and vascular information. MR imaging is still a relatively new technique for imaging the newborn, and very preterm infant in particular, and an active area of research and development. Because scan duration and safety of the often critically ill infants is essential, special emphasis is placed on the development of a safe and quick imaging workflow. Therefore, improvements in equipment (ie, MR-compatible patient monitoring units, incubators, transport systems, dedicated pediatric radio frequency (RF) antennas, and so forth) and MR sequences (ie, parallel imaging and motion-correction methods) are of interest and are often worked on in tandem.
This article reviews the particular needs, equipment, and techniques for MR imaging of the newborn infant and seeks to give an outlook on future technology-driven developments in this area of research.
Practical issues
Risks Associated with Neonatal MR Imaging
Most neonate MR imaging examinations are clinically indicated by injuries and malformations of the brain and spine, such as hypoxic ischemic brain injuries, seizures, trauma, inflicted head injuries, spinal dysraphia, or metabolic diseases, followed by congenital cardiac defects, cardiovascular malformations, and by musculoskeletal indications. All these applications deal with relatively unstable infants, and special management is needed to perform the scan without harming the infant. Potential hazards are acoustic noise, adverse thermal conditions, hemodynamic or ventilation instability, and general risks related to transport and handling. Therefore, close and reliable monitoring of vital functions, support for respiratory and cardiovascular functions, as well as fluid electrolyte and thermoregulatory homeostasis throughout the examination, are warranted. Additional risk factors are sedation or general anesthesia, which can lead to acute and long-term complications. Because the MR examination by itself bears a risk to the ill and unstable infant, comprehensive practice parameters and recommendations have been published for preterm and full-term neonates that specifically address the necessity and timing of the MR examination as a function of the clinical condition of the infant.
MR Safety and Screening
When MR imaging is clinically indicated, the critically ill preterm or term infant usually has to be transported to the MR suite of the radiology department, which in many institutions is far from the NICU. The logistics of transport and imaging are complex and need to be carefully planned. Neonatology staff (ie, NICU nurses and neonatologists experienced in neonatal resuscitation), arespiratory therapist for infants on ventilator support, and radiology staff (ie, MR technologist, MR nurse, and radiologist) need to communicate and cooperate at all times to ensure a safe preparation, transport, and examination. It is important for all involved personnel to be trained in and follow MR safety guidelines, because the strong static magnetic fields and the alternating RF fields constitute MR imaging–specific risks that mandate precautions. The risk associated with the static magnetic field is not limited to the patient and extends to any person (eg, health care professional or accompanying family member) who enters the magnet room. The American College of Radiology (ACR) has published extensive guidelines on MR safety. The safety recommendations by Stokowski and colleagues and Benavente-Fernandez and colleagues highlight issues that are particularly important and relevant to newborn infants.
It is of utmost importance to ensure that no ferrous objects are brought accidentally into the magnet room, where they could become projectiles because of the strong forces of the magnetic field. However, nonmagnetic metals can also be subject to undesired Lenz forces and torque effects, either caused by fast switching gradients or as a result of being moved inside the static magnetic field. Furthermore, the combination of strong magnetic fields and high level of RF power may cause electronic equipment to fail. For these reasons, everybody needs to be screened for metal on or inside the body, including implants and pacemakers, before entering the MR scan room. Common practice requires that screening forms be completed for each individual as part of the screening process. An increasing number of MR imaging sites follow ACR recommendations on the use of ferromagnetic screening devices as an additional safety layer. These devices may be of particular advantage in a setting such as newborn imaging, wherein a large number of individuals and additional equipment has to enter the magnet room.
Equipment used in the scan room should be labeled according to the scheme proposed by the US Food and Drug Administration (FDA) :
MR safe: A square green “MR safe” label indicates that the item is safe to use in the MR environment and poses no known hazard; such items are nonmetallic, nonmagnetic, and nonconducting, such as plastics or wood.
MR conditional: A triangular yellow “MR conditional” label represents items that have been tested and shown to pose no known hazards in a specified MR environment (eg, maximum static magnetic field, gradients, specific absorption ratio [SAR]) in specified conditions of use (eg, certain routing of cables and leads for equipment).
MR unsafe: MR-unsafe items are labeled with a red circle with diagonal red bar, and represent items that are known to pose a clear and direct threat to persons and equipment in the scanner room (ie, ferromagnetic objects) and therefore must not be brought in.
Well-designed magnet rooms have wave guides (wall openings) between the scan room and the operator room, where fiber optic leads, plastic tubes, and other nonconducting leads or hoses can be fed through. The local MR imaging service may help identify wave guides between the technical room and the scan room. These openings enable the use of non–MR-safe monitoring equipment by placing it outside the magnet room and using fiber optics to connect to the patient. No electrically conductive leads should run through these wave guides, because they act as antennas, compromise the RF shielding of the magnet room, and lead to MR image artifacts.
RF Exposure
During a scan, the patient, as well as all the equipment, inside the MR imaging magnet bore are exposed to high-frequency RF fields. Therefore, all cables and electrically conductive materials can potentially act as antennas and attract RF currents. This can lead to tissue heating due to local hot spots in the RF transmit field in the proximity of the equipment and also to elevated surface temperatures of these items. All items inside the MR imaging bore, as well as in the vicinity, have to be specifically designed and tested for safe use inside the RF transmit field of the scanner.
As additional precautions, cables should always be placed away from the patient and run in straight lines and along the shortest route outside of the bore without forming loops. All manufacturer warnings and recommendations should be followed. Any equipment or conductive material that is not needed or not used during the scan should be removed before the examination. All devices used have to be plugged in properly to ensure safe performance. Equipment should not be used if a visual inspection reveals loss of integrity in electrical or thermal insulation.
The RF power applied to the patient is measured as SAR in Watts per kilogram (W/kg). Limits of SAR depend on many factors such as patient age, patient weight, location of patient inside the RF field, and local regulatory legislation. In the United States, the maximum allowable SAR is 3 W/kg (averaged over 10 minutes) for head imaging and 4 W/kg (averaged over 15 minutes) for whole-body imaging. To ensure that SAR limits are not exceeded, the MR imaging machine uses a so-called SAR monitor, which correlates information about MR imaging coils, patient weight, and patient position with real-time measured data relating to RF field and RF power. Because MR imaging scanners are primarily built for adult-size patients, pediatric imaging with its much smaller and lighter patients constitutes a challenge for the underlying SAR model. The user has to ensure that correct patient SAR parameters are entered into the system. It is also advisable to consult the operator’s manual before using coils such as adult head coils, which have not been specifically designed for use with neonates. In some instances, scanners use the information regarding the type of local coil to estimate patient position. It is therefore always advisable to keep MR imaging coils in their intended location on the patient table.
Patient Preparation, Transportation, and Monitoring
As mentioned earlier, the logistics of transporting newborn infants from the NICU to the MR suite and the MR imaging examination are complex and need to be planned carefully. The workflow varies depending on the severity of the illness, the need for sedation, and the availability of an MR-conditional incubator. Detailed guidelines on how to prepare the unsedated critically and noncritically ill infant in the NICU for transport and MR imaging, as well as a list of equipment needed (also see Table 1 ), are given by Mathur and colleagues. For an alternative description of these aspects, including sedation, the reader is referred to van Wezel-Meijler and colleagues, Pennock, and Maalouf and colleagues.
| Equipment | Manufacturer | Location |
|---|---|---|
| MR-compatible ventilator (with CPAP mode) | Airon | Melbourne, FL |
| Allied | St Louis, MO | |
| Bio-Med Devices | Guilford, CT | |
| Draeger Medical | Telford, PA | |
| Smiths Medical | Dublin, OH | |
| SREE Medical Systems/Advanced Imaging Research | Cleveland, OH | |
| VersMed/GE | Piscataway, NJ | |
| MR-compatible infusion pumps (intravenous pumps) | IRadimed | Winter Park, FL |
| Mammendorfer Institut für Physik und Medizin | Mammendorf, Germany | |
| MEDRAD | Warrendale, PA | |
| MR-compatible pulse oximeter with an infant SpO 2 sensor | Invivo | Orlando, FL |
| Memmendorfer Institut für Physik und Medizin | Mammendorf, Germany | |
| MEDRAD | Warrendale, PA | |
| Nellcor/Covidien | Boulder, CO | |
| Nonin | Plymouth, MN | |
| MR-compatible ECG leads | Conmed | Utica, NY |
| Invivo | Orlando, FL | |
| Medicotest/Ambu | Glen Burnie, MD | |
| Head-stabilizing equipment | CFI Medical Solutions | Flint, MI |
| Par Scientific | Houston, TX | |
| Ear protection (ear muffs) | 3M | St Paul, MN |
| Natus | San Carlos, CA | |
| Portable infant warmer | 3M | St Paul, MN |
| Cardinal Health | Dublin, OH | |
| Embrace | San Francisco CA | |
| Temperature monitoring | LumaSense Technologies | Santa Clara, CA |
| Neonatal RF coil | Invivo | Orlando, FL |
| Lammers Medical Technology | Lübeck, Germany | |
| Philips | Best, Netherlands | |
| SREE Medical Systems/Advanced Imaging Research | Cleveland, OH | |
| MR-conditional incubator | Koala System AB | Waxholm, Sweden |
| Lammers Medical Technology | Lübeck, Germany | |
| SREE Medical System/Advanced Imaging Research | Cleveland, OH |
Preparing neonates for MR imaging, whether sedated or unsedated, includes reconnecting the infant to an array of MR-safe or MR-conditional monitoring sensors such as electrocardiogram (ECG), SpO 2 , noninvasive blood pressure (NIBP), temperature, and respiratory sensors. In addition, an MR-compatible ventilator as well as infusion pumps may be used. The patient needs to be protected from noise and must be kept warm during transport and scanning by using sheets, blankets, and maybe also a Porta-Warm infant mattress. Furthermore, patient stabilization devices are needed to reduce motion during the scan. For this purpose, vacuum cushions, foam pads, and straps may have to be applied. Even without sedation, the preparation time for neonates is 30 to 60 minutes.
The main steps and equipment used for transportation of neonates to the MR room are as follows.
Ventilation
A ventilated infant should be transferred to an MR-compatible ventilator with MR-conditional gas cylinders before leaving the NICU. MR-compatible ventilators are available from several manufacturers (see Table 1 ). Alternatively, standard neonatal ventilators can be used. However, they need to be placed outside the magnet room with the extended hoses fed through the wave guide on the filter plate in the MR room.
Intravenous solutions and pumps
The number of intravenous (IV) solutions that need to be administered during MR imaging should be minimized to the absolutely necessary before transport, and IV pumps should be exchanged with MR-compatible pumps, which are available from several manufacturers (see Table 1 ). As with non–MR-safe ventilators, non–MR-safe IV pumps can still be used, but the IV lines need to be extended and fed through the wave guide into the control area outside the scanner room where the IV pumps have to be placed.
Oxygen and heart rate
An MR-compatible pulse oximeter (see Table 1 ), with the probe preferably taped to a foot, should be used to monitor the infant’s oxygen saturation level. Pulse oximeters scan the pulsatile arterial blood during each cardiac cycle to calculate the oxygen saturation, thereby also delivering data about the pulse rate and cardiac output. Hence, pulse oximeters are not only useful to detect hypoxemia and hypoxia but also to measure the real-time heart rate to detect bradycardia and apnea, which may occur particularly in preterm infants. Furthermore, MR-compatible ECG leads (see Table 1 ) may be attached to the infant’s chest before leaving the NICU, to monitor the heart rate more accurately during the MR examination. Monitoring the heart rate during MR imaging is important, because changes to the heart rate have been observed in newborns and linked to the MR examination.
Ear protection
When imaging neonates, noise reduction is essential to protect their sensitive ears from the excessive noise emanating from the MR imaging scanner. Therefore, earplugs and neonatal ear muffs (see Table 1 ) are fitted before the transport. In addition, lightweight headphones may be placed over the muffs in the MR room to further reduce noise.
Temperature
It is important to monitor and maintain the body temperature of the infant during transport and MR imaging. Infant blankets used for a firm swaddle and/or a portable infant warmer together with a warming pad should be used to ensure that the infant stays warm. An MR-compatible fluoro-optic temperature probe (see Table 1 ) should be taped in the NICU to the infant’s abdomen for constant monitoring of body temperature.
Resuscitation equipment
MR-compatible resuscitation equipment should be available during transport and MR examination, including an MR-compatible laryngoscope and endotracheal tubes in various sizes suitable for newborns and a self-inflating AMBU bag. During the MR imaging examination, the resuscitation cart should be kept outside. There should also be a positive-pressure oxygen delivery system and wall suction available outside in close proximity to the MR suite. In an emergency, any resuscitation attempt should be made outside the MR room, because the risk of an accident due to MR unsafe equipment rushed into the room during resuscitation is high.
Incubator
A relatively safe transport of the infant to the MR suite and MR examination is assisted by the use of an MR-compatible incubator with integrated MR-compatible monitoring equipment and neonatal MR coils. In the NICU, the infant is placed into the incubator; earmuffs are attached; intravenous infusions, ventilators, and all necessary MR-compatible monitors are connected; and the RF coils (eg, head or body array) are attached before the infant is transferred to the MR room and scanned within the incubator. Besides offering a safe humidity-controlled and temperature-controlled environment to the infant at all times, the MR-compatible incubator optimizes the workflow because there is no additional transfer of the infant necessary when entering and leaving the MR room.
A commercially available solution, the FDA-approved LMT nomag IC 1.5/3.0 MR-conditional incubator (Lammers Medical Technology, Lübeck, Germany) with integrated MR head coil and optional body array, MR-compatible ventilation, and integrated monitoring has been installed in more than 50 centers worldwide. One of the first incubators of this type was installed at the Children’s Hospital in Los Angeles ( Fig. 1 ) and has been used since for ground-breaking work in structural and functional MR imaging, diffusion, and spectroscopy there and in other institutions.

Custom solutions include the incubator used by the University of California San Francisco to perform pioneering work in neonatal MR imaging and spectroscopy ( Fig. 2 ). This incubator was built around a GE Signa patient table and docked directly to the magnet, thereby eliminating the need for lifting the incubator with the infant inside onto the patient table, in contrast with the LMT incubator, which fits all vendor platforms (eg, GE, Philips, Siemens) but requires lifting. Also, the incubator volume is approximately 3 to 4 times that of the LMT, offering more space for the infant. Other features include a closed-circuit infrared TV camera to monitor the baby, even in total darkness; double walls for added insulation and noise reduction; a massive battery bank that warrants full operation for several hours independent of wall power; thermal management hardware (controller, fan, heating elements, and so forth) that is separated from the incubator and stays outside the magnet; a filling factor optimized birdcage head coil that easily slides out of the way without moving the baby; an alternative mechanism to slide the baby into the incubator without moving the coil; and a preparation area with work lights to prepare the infant for the transport and scan.

If an MR-compatible incubator is not available, a standard transport incubator can be used. However, because the transport incubator is usually MR unsafe, the infant needs to be taken out of the incubator outside the scanner room. It is important that all equipment that is not MR safe is clearly labeled and all involved staff members are instructed to leave MR-unsafe equipment outside the magnet room.
Sedation
Sedation is usually unnecessary for newborn infants undergoing MR imaging, because most infants sleep through the examination after being properly fed, swaddled, and prepared for the scan. In the few neonates who require sedation to prevent motion artifacts, orally administered chloral hydrate is frequently used. An initial dose of 50 mg/kg for infants younger than 3 months is common, but dose levels vary from 25 to 55 mg/kg among institutions. Institutional guidelines are usually in place for the selection and administration of effective and safe sedation during radiological procedures.
Immediate risks introduced by chloral hydrate sedation, such as postsedation apnea, bradycardia, and oxygen desaturation, are associated with disease and indirectly associated with age, and hence are higher in very young and critically ill neonates. Recent concerns have been raised about potential long-term effects such as neurodegeneration, with possible cognitive and behavioral problems. These concerns are based on observations of neurotoxicity of anesthetic agents that show subtle, but prolonged, behavioral changes in rodent models. However, the current evidence is insufficient to recommend substantial changes in clinical practice. Nevertheless, avoiding unnecessary sedation in these patients is recommended. Some institutions have therefore developed procedures and guidelines for MR imaging in neonates without giving sedation, and have been successful in consistently performing safe and high-quality MR examinations by following these procedures.
RF coils for neonatal MR imaging
Maximizing Signal-to-Noise Ratio
Anatomic structures are significantly smaller in infants than in adult patients. For instance, the size of the brain of an average-weight term newborn is approximately 25% that of an average adult ; higher-resolution scans are therefore needed to depict anatomic features in detail. A high signal-to-noise ratio (SNR) is a prerequisite for the increased resolution needed for MR imaging of newborns. SNR is directly proportional to the voxel size: reducing the voxel in all 3 dimensions by 20% decreases the voxel volume and hence the SNR by approximately 50%. Doubling the resolution in all 3 spatial dimensions results in an eightfold reduction in the voxel volume and SNR.
A potential source for additional SNR is the RF receive coil used for imaging. In many sites, adult head or extremity coils are used for scanning infants because of the lack of dedicated neonatal head or body coils. Often, the size of these adult patient coils is significantly larger than that required for infants, which leads to a suboptimal filling factor. The filling factor is a figure of merit for an MR imaging coil: a coil with a higher filling factor produces better SNR than a coil with a lower filling factor, when other protocol parameters and patient load remain unchanged. The concept of filling factor was a first attempt to explain the inherent SNR property of a coil. It has since been replaced by a more general concept of coil sensitivity, using the principle of reciprocity from the antenna theory.
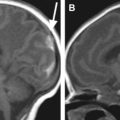
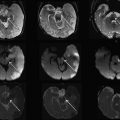
Dedicated coils tailored to the size of the neonate have been developed over the years in an attempt to bring the coil closer to the infant. Fig. 3 shows a neonate birdcage coil for use with the LMT incubator and a size comparison with standard birdcage head coils used for adults. Fig. 4 compares the T2-weighted images acquired with the standard birdcage (see Fig. 3 , coil 4) and the neonate birdcage (see Fig. 3 , coil 1) in the same infant. The higher SNR achieved with the neonate birdcage coil, approximately a factor of 3, was traded for resolution and acquisition speed: the image shown in Fig. 4 B had an 8-times higher spatial resolution and half the acquisition time compared with that shown in Fig. 4 A.

The sensitivity of an MR imaging coil array improves not only when the array is moved closer to the patient but also when the size of the individual coil elements that form the array are optimized. In general, coil arrays perform better if they can be adjusted in size, particularly in the anterior-posterior direction, to accommodate a larger patient population (eg, from very low birth weight premature infants to 6-month-old regular-weight infants). Another issue with adult-sized coils is that they are poorly matched to the smaller patient load when used with infants, which enhances the noise contributions from the electronics inside the coil. A dedicated pediatric coil array is better matched to neonates and therefore minimizes the image noise generated by the coil.
Following these basic principles of coil design (ie, maximizing SNR by closely fitting the coils around the patient and minimizing intrinsic losses caused by coil hardware), more pediatric array coils have become available in recent years. A representative set of commercially available solutions is shown in Fig. 5 . However, except for the integrated head-neck-spine-abdomen coil array shown in Fig. 5 A, these coils are optimized for an older pediatric population and not for preterm and term infants, but they can still be used and offer an SNR benefit compared with adult phased array coils. In addition, they are also not compatible with the LMT incubator, which has its own current line of RF antennas with up to 12 elements. Table 1 summarizes current commercially available pediatric coils suitable for neonate imaging.
Advanced RF Coils
A significant challenge for hardware design in MR imaging in children is the growth of patients and the large variation in patient size. As per growth charts, the average head circumference is 20 to 22 cm for preterm infants at 22 weeks, 38.5 cm for full-term newborns, and up to 46 cm (95 percentile) for 6-month-old babies. A single infant head coil covering the entire size range is optimal, but the large variation in head size impedes meeting this goal. Flexible or adjustable coil arrays could be used for wider age ranges. However, there are limits in applying flexible designs to volume coils such as head coils. In addition, adjustable coils, with their more complex mechanical solutions, are not as reliable and easy to clean, the latter limitation being important in the context of very young patients. A comprehensive set of pediatric MR imaging coils should ideally contain flexible or adjustable coils for imaging of the torso/spine region, and a set of differently sized head coils.
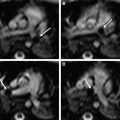
Recent research has focused on the development of 32-channel brain arrays for pediatric neuroimaging spanning the age group of neonates to 7-year-old children ( Fig. 6 ). This development is a logical continuation of the success of the 32-channel brain arrays that have become standard for highest quality clinical neuroimaging in adults because of their SNR benefits and ability to obtain exquisite images in reduced acquisition time by using parallel imaging techniques. The coils shown in Fig. 6 consist of 2 parts: a deep posterior segment laid out like a soccer ball and a separate, detachable paddle with slightly larger loops that covers the forehead ( Fig. 7 ). The head rests deep in the posterior segment and head movement is constrained by this shape. SNR comparisons in age-matched phantom models of the head among the set of 32-channel pediatric coils, a 32-channel adult head array, and a pediatric-sized birdcage are shown in Fig. 8 . The SNR of the neonate 32-channel coil is 3.6 times higher at the periphery (edge of the phantom) and 1.25 times higher at the center of the phantom than that of the adult 32-channel head coil. When averaged over the whole brain, the SNR of the neonate coil is still 2.8 times higher than that of the adult array. The SNR of the neonate array is 5.4 times higher at the periphery and 1.3 times higher at the center than that of the birdcage coil. The high number of coil elements combined with the high SNR significantly reduces the scanning time, which is of utmost importance in neonates. Furthermore, the likelihood of head motion is reduced during shorter scans and because of the coil design, which is an essential prerequisite for accurate functional imaging (blood oxygenation level dependent, diffusion) studies that are required during diagnostic assessment of ill preterm or term infants.