Testicular ultrasound is typically the first-line imaging examination in evaluating scrotal pathology. However, MR imaging can often provide valuable additional information, especially when ultrasound and/or clinical examinations are inconclusive. This is particularly evident when encountering testicular or paratesticular lesions, where accurate localization and characterization are paramount for management and prognosis. After reviewing normal scrotal anatomy as seen on MR imaging and offering a sample imaging protocol, the article describes specific indications for scrotal MR imaging and highlights imaging findings unique to various benign and malignant causes.
Key points
- •
Scrotal MR imaging provides valuable information when clinical and/or ultrasound evaluations are inconclusive.
- •
Specific advantages include more accurate lesion localization and characterization.
- •
Distinction between testicular and paratesticular location is crucial for management and prognosis.
- •
Certain imaging features on MR imaging help in differentiating benign versus malignant lesions, as well as in distinguishing between specific malignant histologies.
- •
MR imaging is accurate in local staging of testicular neoplasms.
Introduction
Imaging evaluation for suspected scrotal pathology should commence with ultrasound (US), owing to its low cost, widespread availability, lack of ionizing radiation, and its high sensitivity in the detection of scrotal masses. However, MR imaging provides valuable information if US and/or clinical examination findings are equivocal or incongruent. One study of 26 patients who underwent MR imaging after an inconclusive US, reported that MR imaging provided additional and correct information in 23/26 patients (82.1%). In another study of 34 patients with an inconclusive clinical and US evaluation, the leading MR imaging diagnosis was correct in 31/34 patients (91% vs 29% for the leading correct diagnosis on the inconclusive US), improving management for both the urologist and urologic oncologist. This study also reported that the use of MR imaging in this setting yielded cost savings for the referring physician (ranging from $530 to $730 per patient) and the patient ($3833 per patient originally scheduled for surgery).
Specific advantages of MR imaging in the setting of a suspected scrotal neoplasm include accurate lesion localization and characterization. Accurate lesion location as intratesticular or paratesticular is critical, as the latter is most often benign and can obviate the need for a radical orchiectomy. Owing to its wider field of view, several studies have shown MR imaging to be excellent in this setting, particularly when the scrotum is markedly enlarged. The inherent soft tissue resolution capabilities of MR imaging enable accurate identification of lesions containing blood, fat, and/or fibrous tissue and allow for the detection of subtle enhancement that may be missed on US, leading to more accurate characterization of intra- and paratesticular masses. MR imaging has also been shown to be accurate in the local staging of testicular neoplasms, which is of particular importance in patients considered for organ-sparing surgery.
Following a review of normal MR imaging scrotal anatomy and imaging technique, the following review will elucidate specific indications in which MR imaging is useful in the evaluation of intra- and extratesticular tumors. For extratesticular masses, this includes accurate lesion localization and characterization. For intratesticular masses, this includes distinguishing benign masses and pseudotumors from malignant neoplasms, differentiating between germ cell tumors (GCTs) (seminomas vs nonseminomatous tumors) and between GCTs and nongerm cell tumors, and evaluating local spread of disease.
Normal anatomy and imaging technique
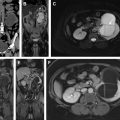
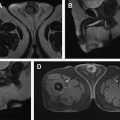
Normal adult testes are well-defined ovoid structures that demonstrate hyperintense T2 signal and low to intermediate T1 signal ( Fig. 1 A, B ). After administration of intravenous contrast, both testes demonstrate moderate homogenous enhancement with a gradual increase in signal on dynamic contrast-enhanced imaging ( Fig. 1 C). On diffusion weighted imaging (DWI), normal testicle parenchyma is homogenously hyperintense ( Fig. 1 D).

The tunica albuginea manifests as a thin hypointense band on all imaging sequences and is indistinguishable from the visceral layer of the tunica vaginalis (see Fig. 1 A, B). The visceral and parietal layers of the tunica vaginalis may be separated by a variable amount of T2 hyperintense fluid. The mediastinum testis appears as T1 and T2 hypointense band extending along the long axis of the testicle (this is better seen on the T2-weighted sequences owing to the inherent hyperintense T2 parenchymal signal) ( Fig. 1 E). The rete testis may manifest as T2 hypointense bands or thin enhancing bands on T1 postcontrast sequences, extending from the mediastinum testis to the tunica albuginea.
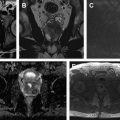
The epididymis is T2 hypointense and T1 isointense with respect to the testicular parenchyma. On postcontrast sequences, the epididymis is hyperintense with respect to the testicular parenchyma ( Fig. 2 ). The scrotal wall is typically hypointense on both T1- and T2-weighted sequences.

`The biological effects of the magnetic field in the MR environment on spermatogenesis are not clearly understood. One study concluded that a 30-minute exposure to a 1.5 Tesla (T) static magnetic field resulted in some deleterious effects on spermatogenesis in mice. Another study noted that MR imaging at high specific absorption rates (mean 0.72 W/kg, average imaging time 23 minutes) resulted in statistically significant increase in average scrotal temperature (up to 3.0°C). However, these temperatures were noted to be below the level known to affect spermatogenesis in humans. Therefore, as with any imaging study, it is imperative to weigh the risks and benefits before proceeding with the MR examination.
All MR examinations of the scrotum are monitored by the radiologist to ensure adequate coverage. The patient is placed supine with feet first in the MR imaging unit. The penis should be raised, covered in gauze, and taped to the lower abdominal wall. In order to elevate the scrotum, a folded towel is placed between the upper thighs. An additional towel is placed over the scrotum and penis with the coil placed on top of the second towel. Either a circular surface coil or a phased-array multichannel coil can be used. Scrotal MR examinations at the authors’ institution are performed on either a 1.5- or a 3.0-T magnet system, with the latter allowing for higher signal-to-noise ratio.
A sample MR imaging protocol is provided in Table 1 . After obtaining localizer sequences, small field-of-view T2-weighted turbo spin echo (TSE) sequences with thin sections are performed in the axial, coronal, and sagittal planes. Subsequently, a T2-weighted TSE fat-saturated sequence is performed through the scrotum in order to detect subtle regions of T2 hyperintensity and to depict fluid collections and edema. The use of fat saturation is also helpful in detecting macroscopic fat. By default, this is performed in the sagittal plane, although axial and/or coronal images may be used if the findings are better depicted in these planes. Thereafter, the authors routinely perform axial T1-weighted dual-echo spoiled gradient echo (GRE, both in phase and out of phase) sequences to detect microscopic lipid and/or blood products. Some institutions perform additional T1-weighted TSE images in one or multiple planes due to their higher spatial resolution and increased signal-to-noise ratio.
| Imaging Plane and Sequence | Section Thickness (mm) | Gap (mm) | Matrix Size | TR/TE (msec) |
|---|---|---|---|---|
| Sagittal, axial, and coronal localizers | 10 | 15 | 128 x 256 | 15/5 |
| Sagittal, axial, and coronal T2W turbo spin-echo (TSE) | 3 | 0.3 | 192 x 256 | 4420/126 |
| Sagittal T2W TSE fat-saturated a | 3 | 0.3 | 256 x 179 | 4660/143 |
| Axial T1W dual-echo spoiled GRE (in phase and out of phase) | 8 | 10 | 256 x 190 | TR = 212 TE (in phase) = 5.2 TE (out of phase) = 2.3 |
| Axial unenhanced and contrast-enhanced T1W fat-saturated 3D volume GRE | 1.1 | 0 | 256 x 256 | 5.6/2.7 |
| Axial contrast-enhanced T1W fat-saturated 3D volume GRE (large field-of-view) | 3 | 0 | 256 x 135 | 5.0/2.4 |
| Axial DWI (ADC generated using the highest b value) | 3 | 0.5 | 116 x 116 | 4300/63; b value of 50, 400, and 800 s/mm 2 |
a Default plane is sagittal although this should be monitored and performed in the plane that best depicts the pathology.
Axial T1-weighted fat-saturated 3-dimensional (3D) volumetric GRE sequences are subsequently performed, both before and after the administration of a gadolinium-based contrast agent, in order to detect enhancement (with the postcontrast images performed 1 minute following contrast injection). Obtaining 3D volumetric GRE sequences allows for rapid imaging acquisition with an isotropic data set that can be reconstructed with equal resolution in additional planes if needed. In order to fully appreciate the extent of the pathologic process, the authors routinely perform an additional large field-of-view T1-weighted fat-saturated 3D volumetric GRE sequence extending from the inferior aspect of the kidneys to the scrotum. Postprocessed subtraction images (precontrast images subtracted from contrast-enhanced images) are provided and are particularly useful for lesions that demonstrate inherent hyperintense T1 signal.
Several studies have investigated the use of dynamic contrast-enhanced (DCE) sequences in scrotal MR imaging. A study in 2013 concluded that DCE MR imaging may be used to differentiate malignant from benign intratesticular masses, with the former demonstrating rapid enhancement and gradual washout and the latter exhibiting similar initial imaging features (although time to peak enhancement was delayed) followed by either a plateau or a slower increase in enhancement. More recently, one study concluded that DCE may be used to distinguish between benign and malignant nonpalpable intratesticular tumors and reported some DCE parameters that may differentiate seminomas from Leydig cell tumors. A sample protocol for DCE imaging provided by the European Society of Urogenital Radiology is provided in Table 2 .
| Imaging Plane and Sequence | Thickness | Contrast Timing | Imaging Duration |
|---|---|---|---|
| Coronal 3D fast field-echo sequence | 4 mm with 2 mm overlapping sections | Bolus injection (1–2 mL/s) |
|
Some studies have also demonstrated the utility of DWI in scrotal MR imaging, reporting lower apparent diffusion coefficient (ADC) values in seminomas versus nonseminomatous tumors, a finding that may aid in their differentiation. Several studies have also shown that the use of high b value DWI, when combined with conventional MR sequences, is associated with increased accuracy in characterizing scrotal masses and detecting nonpalpable cryptorchidism over using DWI or conventional MR sequences alone. A study in 2014 reported lower ADC values in the ipsilateral testicular parenchyma of patients with varicoceles compared with healthy control volunteers, concluding that this may be used as a diagnostic indicator of fibrosis. At the authors’ institution, DWI images are performed in the axial plane with an eco planar diffusion pulse sequence using b values of 50, 400, and 800 s/mm 2 . ADC maps, generated on the MR console using the highest b value, are provided for assessment.
Imaging findings/pathology
Extratesticular Masses
- •
Lesion localization and characterization:
Although US remains the first-line imaging modality in compartmentalizing scrotal masses, MR imaging may be helpful if findings are inconclusive. This problem can arise when the relationship of the lesion to the hyperechoic tunica albuginea on sonography is difficult to establish. In addition, MR imaging provides for wider field-of-view imaging, allowing complete assessment of lesions that may only be partially imaged using US.
Several studies have demonstrated the utility of MR imaging in localizing scrotal masses and delineating their relationship to surrounding structures. In one study, all 23 scrotal lesions (20 intratesticular, 3 extratesticular) were correctly localized by MR imaging. In another recent study, MR imaging demonstrated high accuracy in distinguishing intra- and extratesticular masses, appropriately localizing all 80 scrotal lesions (28 intratesticular and 52 extratesticular).
The superior soft tissue resolution characteristics of MR imaging, in conjunction with lesion location and morphology, may also aid in the characterization of extratesticular scrotal masses. For aggressive paratesticular neoplasms, MR imaging can depict the local extent of disease and detect the presence or absence of metastases. Diffusion-weighted imaging may also be of utility with one study reporting hypointense DWI signal with increased ADC (1.72 ± 0.60 × 10−3 mm 2 /s) favoring benignity in paratesticular lesions.
Accurate localization and characterization are imperative because most extratesticular lesions are either benign or mimic neoplasms, obviating the need for radical orchiectomy. Examples of benign lesions include epididymal cystic lesions (cysts or spermatoceles), tunica albuginea cysts, inflammatory conditions (epididymitis), fluid collections (hydroceles, pyoceles), varicoceles, polyorchidism, or hernias. Primary solid paratesticular tumors are uncommon and can range from benign lesions, such as lipoma, adenomatoid tumor, fibrous pseudotumor, and cellular angiofibroma, to malignant neoplasms, such as spermatic cord sarcoma and metastases ( Table 3 ).
| Extratesticular Mass | Clinical Features | MR Imaging Appearance |
|---|---|---|
| Lipoma |
|
|
| Adenomatoid tumor |
|
|
| Fibrous pseudotumor |
|
|
| Cellular angiofibroma |
|
|
| Sarcoma |
|
|
| Metastases |
|
|
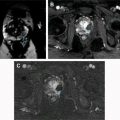
Paratesticular lipomas are the most common benign extratesticular masses, often arising in the spermatic cord. Although most lipomas are hyperechoic on US, the echotexture can be variable, likely related to the number of internal interfaces. MR findings are diagnostic, demonstrating a nonenhancing mass with identical signal intensity to fat on T1- and T2-weighted images ( Fig. 3 ). Chemical shift artifact will be present at any water-fat interfaces associated with the lipoma.

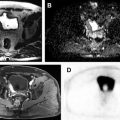
Adenomatoid tumors are the second most common extratesticular masses, often arising from the epididymal tail. Lesions are typically unilateral and occur more frequently on the left side. Adenomatoid tumors are often diagnosed between the ages of 20 and 50 years, and while most often an incidental finding, they may alternatively manifest as a painless testicular mass. In approximately 30% of patients, adenomatoid tumors can present with pain. Although both US and MR imaging findings are nonspecific, MR imaging can be more definitive about the paratesticular location of the mass allowing for a more confident diagnosis. Adenomatoid tumors have been reported to be T2 hypointense with respect to the testicular parenchyma. Postcontrast imaging appearance may be hypo- or hypervascular, possibly related to the amount of associated granulation tissue ( Fig. 4 ).

Although uncommon, fibrous pseudotumors (FPTs) are the third most common extratesticular masses, following lipomas and adenomatoid tumors. In one case series, FPTs were found in 7/114 paratesticular tumors (0.061%). This lesion is not a true neoplasm, but rather represent a benign fibrous proliferation of paratesticular tissue, most often occurring in the tunica vaginalis, followed by the epididymis, spermatic cord, and tunica albuginea. Clinically, FPTs manifest as a firm nodule or diffuse nodular thickening of the scrotum with an antecedent history of trauma or infection found in 30% of patients. MR imaging can allow for accurate paratesticular localization as well as characterization, with FPTs often demonstrating markedly hypointense T1 and T2 signal with variable enhancement on postcontrast imaging sequences ( Fig. 5 ).

Cellular angiofibroma is a rare, benign neoplasm, composed of spindle-shaped cells, a collagen-rich myxoid stroma, and small- to medium-sized blood vessels. The presence of intratumoral fat has been reported in 24% to 56% of cases. It is usually found in patients during the fifth to eighth decade of life and most often arises in the inguinal region, followed by the scrotum and perineum. Clinically, patients present with a painless, slow-growing scrotal mass with one study reporting a size range of 3.0 to 25.0 cm (mean 7.9, median 7.0 cm). Although MR imaging findings are nonspecific, they have been noted to follow the histologic appearance of this neoplasm. Owing to its myxoid stroma and numerous vessels, cellular angiofibromas demonstrate heterogeneously hyperintense T2 signal with heterogeneous enhancement on postcontrast imaging ( Fig. 6 ). If present, intratumoral fat can also be detected on MR imaging. On DWI, cellular angiofibromas demonstrate no area of restricted diffusion.

Sarcomas are the most common malignant masses of the spermatic cord. Histologic subtypes include rhabdomyosarcoma, liposarcoma, leiomyosarcoma, malignant fibrous histiocytoma, fibrosarcoma, and undifferentiated sarcoma. Of these, rhabdomyosarcoma is the most common (40% of cases), followed by liposarcoma and leiomyosarcoma. Although most spermatic cord sarcomas occur in older patients, rhabdomyosarcoma frequently occurs in children and young adults. Sarcomas most often manifest as a painless and hard inguinal/scrotal mass, although malignant fibrous histiocytoma has been reported to present with pain. MR imaging is useful for preoperative planning and confirming the relationship of the mass to adjacent structures (such as the testicle and vasculature). Although most sarcomas have a nonspecific appearance, demonstrating heterogeneous enhancement with variable amounts of internal hemorrhage and necrosis, MR imaging may detect the presence of fat within the lesion suggesting the diagnosis of liposarcoma ( Fig. 7 ).

Paratesticular metastases are rare, with less than 8% of epididymal neoplasms representing metastases. The most common primary tumor sites associated with metastases to this region are (in decreasing order of frequency) prostate gland, kidney, stomach, colon, carcinoid tumor of the ileum, and pancreas.
Intratesticular Masses
- •
Differentiating benign masses and pseudotumors from malignant masses:
MR imaging of the scrotum has been shown to accurately differentiate benign from malignant intratesticular masses in cases where US and/or clinical findings are inconclusive. Preoperative diagnosis of benign intratesticular lesions avoids unnecessary radical orchiectomy, with alternative treatment strategies such as follow-up, biopsy, tumor enucleation, or testis-sparing surgery potentially justified. Examples of such lesions include intratesticular hematoma, tubular ectasia of the rete testis, epidermoid cyst, and segmental (or diffuse) testicular infarction.
One study of 34 patients reported an MR accuracy rate of 91% in distinguishing benign from malignant testicular lesions after inconclusive clinical and US evaluations. In a study of 17 cases of tumors or pseudotumors in whom an MR imaging was performed after an inconclusive US, 15/17 were reliably characterized with the negative predictive value of characterizing a benign lesion on MR imaging reported as 100%. Another group demonstrated high sensitivity (100%), specificity (87.5%), and accuracy (96.4%) in differentiating benign from malignant intratesticular masses using MR imaging.
Absence of contrast enhancement within intratesticular masses has been described as the most sensitive sign for predicting benignity. However, in cases where benign lesions enhance (eg, in inflammatory conditions such as epididymo-orchitis), DCE and DWI imaging may be useful for further characterization. The DCE features of benign lesions in one study were reported as strong enhancement with a late peak followed by either a plateau or a gradual increase in signal intensity. Benign lesions have been reported to demonstrate no restricted diffusion with higher ADC values than malignant intratesticular masses. Using a cut-off value equal or less than 0.99 × 10−3 mm 2 /s, one study showed high sensitivity (93.3%), specificity (90.0%), positive predictive value (87.5%), and negative predictive value (94.7%) in characterizing malignant intratesticular masses. Another study reported a mean ADC value of 1.56 ± 0.85 × 10−3 mm 2 /s for benign intratesticular lesions ( Table 4 ).