Pre- and postoperative evaluation, serial follow-up studies, and screening examinations of the aorta are performed with noninvasive cross-sectional imaging modalities like CT and MR imaging. MR imaging allows for dedicated comprehensive evaluation without exposure to iodinated contrast or ionizing radiation. The additional advantage of MR imaging is that it can provide not only morphologic but also functional information. The purpose of this article is to advance knowledge and understanding of MR imaging techniques and their application to common aortic pathologies.
Key points
- •
MR imaging allows for comprehensive evaluation of thoracic aorta without exposure to iodinated contrast or ionizing radiation.
- •
Basic knowledge of pulse sequences and common artifacts and ways to avoid them is essential for successful MR imaging of the aorta.
- •
Noncontrast magnetic resonance angiography (MRA) of the aorta can adequately answer clinical questions, even in scenarios where gadolinium administration is contraindicated.
Introduction
Invasive imaging preceded noninvasive cross-sectional imaging in evaluation of thoracic aorta. Recently, advances in CT and MR imaging have significantly improved knowledge, understanding, and management of thoracic aortic diseases. Iodinated contrast-enhanced (CE) CT is readily available, provides superior spatial resolution, and constitutes the cornerstone for pre- and postoperative evaluation of the thoracic aorta. MR imaging provides morphologic and functional information without utilization of iodinated contrast or radiation exposure. MR imaging is, therefore, preferred in younger patients in whom annual follow-ups are required.
This article discusses MR imaging techniques, protocols, imaging planes, and application of MR imaging in the assessment of common thoracic aortic pathologies, such as aneurysms, aortic dissection, penetrating ulcers, congenital anomalies, and postoperative complications. Imaging artifacts that potentially lead to misdiagnosis and ways of overcoming these artifacts also are discussed.
Introduction
Invasive imaging preceded noninvasive cross-sectional imaging in evaluation of thoracic aorta. Recently, advances in CT and MR imaging have significantly improved knowledge, understanding, and management of thoracic aortic diseases. Iodinated contrast-enhanced (CE) CT is readily available, provides superior spatial resolution, and constitutes the cornerstone for pre- and postoperative evaluation of the thoracic aorta. MR imaging provides morphologic and functional information without utilization of iodinated contrast or radiation exposure. MR imaging is, therefore, preferred in younger patients in whom annual follow-ups are required.
This article discusses MR imaging techniques, protocols, imaging planes, and application of MR imaging in the assessment of common thoracic aortic pathologies, such as aneurysms, aortic dissection, penetrating ulcers, congenital anomalies, and postoperative complications. Imaging artifacts that potentially lead to misdiagnosis and ways of overcoming these artifacts also are discussed.
MR imaging techniques
MR imaging of thoracic aorta is commonly used for interrogation of a variety of genetic, traumatic, atherosclerotic, inflammatory, and idiopathic disease processes. Although gadolinium-based contrast material is frequently used for thoracic aortic assessment, depending on the clinical scenario and patient specific factors, the clinical question about specific aortic pathology can be adequately answered even without gadolinium as needed. This section focuses on various MR sequences and their applications.
Black Blood Imaging
Black blood (BB) images of blood vessels are acquired with double-inversion recovery prepulse technique. This flow-sensitive technique uses 2 180° inversion recovery prepulses to null the signal of flowing blood:
- •
The first prepulse is not slice selective and inverts the longitudinal magnetization vector (Mz) in the entire body.
- •
The second prepulse is slice selective and inverts Mz back to its original orientation but only in the selected slice.
The excitation pulse is applied when Mz of blood that was initially outside the imaging slice has relaxed to zero, therefore not generating any signal. If there is flow, this blood from outside has replaced the blood in the imaging slice, resulting in nulled (dark) blood pool signal.
For routine BB imaging of the thoracic aorta, the authors use a vectorcardiogram (VCG)-gated proton density–weighted 2-D fast spin-echo (FSE) sequence with a slice thickness of 6 to 8 mm. The routine protocol includes a stack of 6- to 8-mm slices in the transverse plane of the chest and in the sagittal oblique (candy cane) view of the aortic arch. VCG gating increases imaging time but, however, significantly reduces motion artifact. Single-shot FSE (SSFSE) sequences are much faster than basic FSE sequences and allow for acquisition of the entire imaging stack in a single breath-hold. In the acute setting, it is recommended to acquire T1-weighted BB images with fat saturation, to increase the conspicuity of aortic wall hematoma. T2-weighted BB imaging with fat saturation can demonstrate aortic wall or periaortic edema in inflammatory disorders.
Despite being a robust and established technique, BB images are prone to artifact. Common issues are incomplete nulling of the blood pool, particularly if flow is aligned with the imaging plane, which is commonly encountered with imaging of the descending aorta in the candy cane plane and can be alleviated by decreasing the slice thickness of the second (slice-selective) inversion recovery prepulse (BB slice thickness) ( Fig. 1 ). Another issue is incomplete visualization of the vessel wall, which can be resolved by increasing the BB slice thickness.
Bright Blood Imaging
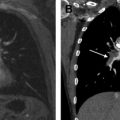
VCG-gated cine acquisitions with 2-D steady state free precession (SSFP) sequences generate time-resolved images, which help not only depict the anatomy of thoracic aorta ( Fig. 2 ) but also visualize flow jets, without use of gadolinium-based contrast material. Signal intensity of the blood pool on SSFP images is inherently high and relatively independent from inflow effects, which is explained by T2/T1-weighted image contrast, which is particularly high for blood and all fluids. 2-D SSFP cine imaging is used to evaluate aortic size, depict aneurysms, and demonstrate dissection flaps and any filling defects, such as intraluminal thrombus. Cine 2-D SSFP is particularly useful for dynamic visualization of the aortic root, aortic valve morphology and motion (see Fig. 2 ), and aortic valve stenosis and regurgitation. The aortic root is best evaluated in multiple planes, including long-axis planes of the left ventricular outflow tract (LVOT) and a stack of consecutive true short-axis images of the aortic root from the aortic annulus to the sinoaortic junction (see Fig. 2 ). The remainder of the thoracic aorta is best evaluated with images in the transverse plane of the chest and in the sagittal oblique view of the aortic arch (see Fig. 2 ). The authors routinely acquire cine images with 6- to 8-mm slice thickness and 25 to 30 reconstructed phases per heartbeat usually during breath-hold. Images can also be acquired during free breathing, by averaging multiple acquisitions.
Angiographic MR imaging of the vasculature without gadolinium is becoming increasingly popular and is indispensable in patients with renal failure in whom both iodinated and gadolinium-based contrast material are contraindicated precluding CE-CT and gadolinium-enhanced MRA. For noncontrast MRA of the thoracic aorta, traditional techniques, such as time-of-flight, have been replaced by newer methods. The most commonly used contemporary method is VCG- and respirator navigator–gated 3-D SSFP (see Fig. 2 ), allowing for near isotropic high spatial resolution imaging of the thoracic aorta and its branches, including the proximal coronary arteries, with minimal motion artifact. The inherent high fluid signal of the SSFP sequence provides excellent bright blood contrast, which is further improved by a T2 preparatory pulse to improve contrast between coronary arteries and myocardium. Other advanced noncontrast MRA techniques, such as VCG-gated FSE and arterial spin labeling, aim to suppress venous and background signal and are most beneficial for imaging of the peripheral vasculature. They are not routinely used for imaging of the thoracic aorta, where venous contamination is not usually an issue and suppression of background signal is often not necessary.
Both cine 2-D SSFP and 3-D SSFP sequences provide consistent image quality. When prescribing the VCG- and respirator navigator–gated 3-D SSFP, it is important to tailor the beginning and duration of the acquisition to the diastolic rest period, to avoid image blurring secondary to cardiac motion. This sequence can be affected by arrhythmia and erratic breathing, resulting in lengthy acquisitions, and suffers from low signal-to-noise ratio particularly at small voxel sizes. Despite being a noncontrast MRA technique, when this sequence is used after gadolinium administration, the signal-to-noise ratio and, therefore, image quality are significantly improved. SSFP sequences in general are susceptible to magnetic field inhomogeneity, particularly at higher field strength, which can result in off-resonance artifacts. Sternotomy wires are rarely problematic but embolization coils can render SSFP images nondiagnostic. Both cine 2-D SSFP and 3-D SSFP imaging are less useful for imaging of the aortic wall, which is better appreciated with BB or CE techniques.
Phase-Contrast Imaging
Phase-contrast imaging is used for quantitative flow velocity mapping, usually as part of a comprehensive MR imaging of the heart and aorta in patients with valvular or congenital heart disease. Phase contrast is achieved by applying bipolar gradients to generate phase shifts in moving protons, proportional to their velocity. Phase-contrast images display those phase shifts (velocities) for each pixel, using a gray scale, where midgray indicates zero velocity. Directional information is encoded in the color where white pixels represent velocities in the flow encoding direction and black pixels represent velocities in the opposite direction. An important consideration when performing phase-contrast imaging is setting an appropriate peak velocity encoding value (VENC). Ideally, VENC should be set slightly higher than the peak velocity being measured. Because peak velocities are not known a priori, a common pitfall is using a VENC that is too low, resulting in aliasing or velocity wraparound ( Fig. 3 ). In this situation, the phase shift exceeds 180° and the faster flows are not appropriately represented. Although it is important to have a VENC higher than expected peak velocity, too high a VENC is not desirable because it results in less accurate measurements. For dynamic measurement of velocities during the cardiac cycle, the authors use a VCG-gated cine 2-D acquisition, with approximately 40 reconstructed phases per heartbeat. The most common application is measurement of through plane flow, which is obtained using commercial software by drawing regions of interest around vessel lumen and integrating flow perpendicular to the imaging plane over the duration of the cardiac cycle. Clinical uses are quantification of aortic valve regurgitation, determination of pulmonary to systemic flow ratio (Qp/Qs) in patients with shunts, and evaluation of aortic coarctation. For research purposes, measures of aortic stiffness, such as aortic pulse wave velocity, can be assessed.
Advanced Applications
4-D flow MR imaging is a time-resolved 3-D phase-contrast acquisition that helps evaluate blood flow patterns. This technique is currently investigational and has been used in the thoracic aorta to study flow features in various settings, such as in patients with Marfan syndrome.
Contrast-Enhanced Magnetic Resonance Angiography
CE-MRA acquires angiographic MR images of the thoracic aorta. CE-MRA is traditionally performed during first pass of a bolus of intravenously injected gadolinium-based contrast material. More recently, techniques to acquire high-quality CE-MRA during the equilibrium phase have become available (described later).
First-Pass Contrast-Enhanced Magnetic Resonance Angiography
First-pass CE-MRA, an established technique, is used for a broad range of clinical applications, including evaluation of aortic aneurysms, dissection, and postoperative anatomy. The strengths of this method are high signal-to-noise ratio and accurate depiction of the aortic lumen and its branches, including small vessels. A low-molecular extracellular gadolinium chelate is administrated intravenously using a power injector, with a dose of 0.1 mmol gadolinium/kg body weight. Images are acquired with a T1-weighted 3-D spoiled gradient-recalled echo (SPGR) sequence, usually during breath-hold. Exact timing of the acquisition to the short time period of maximum aortic enhancement is crucial and can be achieved either with test bolus or with monitoring of the contrast bolus. Isotropic 3-D SPGR acquisition allows for reformatting of source images in multiple planes, maximum intensity projections (MIPs), shaded surface display, and volume rendering (see Fig. 2 ; Fig. 4 ). First-pass CE-MRA is, however, not well suited for evaluation of the aortic root because of the short time window available for image acquisition, which precludes cardiac gating. Also, this technique is contraindicated in patients with anaphylaxis to gadolinium and in patients with impaired renal function (an important risk factor for nephrogenic systemic fibrosis).
Contrast-Enhanced Magnetic Resonance Angiography with Gadolinium-Based Blood Pool Contrast Agents
Gadolinium-based contrast agents with increased T1 relaxivity and longer plasma half-life (blood pool agents) extend the time window for CE-MRA image acquisition. The first Food and Drug Administration–approved gadolinium-based blood pool agent is gadofosveset. Gadofosveset can be used for first-pass CE-MRA similar to conventional contrast agents (see Fig. 4 ). In addition, long duration of the equilibrium phase allows for delayed imaging with increased spatial resolution and with cardiac gating, which is particularly useful for imaging of the aortic root and coronary artery imaging. A VCG- and respiratory navigator–gated inversion recovery prepared 3-D SPGR sequence can be used to generate near isotropic angiographic images of the thoracic aorta and the coronary arteries, with high spatial resolution and minimal cardiac motion. Gadofosveset-enhanced MRA during the equilibrium phase has been shown in some preliminary studies superior to first-pass CE-MRA and superior to CE CT in evaluation of endoleak in patients with aortic endografts (see Fig. 4 ).
Recent Advances
Time-resolved CE-MRA demonstrates dynamic enhancement of blood vessels similar to conventional angiography. Images are acquired with a 3-D SPGR sequence, similar to conventional CE-MRA. Parallel imaging and keyhole data sampling are used for accelerated image acquisition necessary for the time-resolved scan. Time-resolved CE-MRA is an invaluable technique for comprehensive evaluation of the thoracic vasculature, including aorta and systemic venous and pulmonary vessels in patients with congenital heart disease.
Imaging planes
A comprehensive approach to evaluation of thoracic aorta includes obtaining 2-D sequences in specific planes that enable detailed assessment of the aorta as well as aortic valve and cardiac morphology and function, as necessary. A sagittal oblique plane parallel to the aortic arch, also called candy cane view, provides exquisite demonstration of the arch along with ascending and descending thoracic aorta in a single plane (see Fig. 2 ), which is important when the clinical question entails assessment of aortic dissection and relationship of the arch vessels to the flap, aortic aneurysm, or other subtle arch abnormalities. The LVOT views (3-chamber and coronal oblique plane along the LVOT) ( Fig. 5 ) provide information regarding aortic valve function, allow qualitative assessment of associated ventricular changes, and help plan phase-contrast imaging for the aortic valve. The true short-axis plane of the aortic valve is particularly helpful in determining the morphologic anatomy of the aortic valve (see Fig. 5 ) and in assessing patterns of commissural fusion in bicuspid valves.