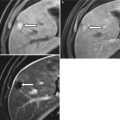
Fig. 15.1
Normal anatomy of the pelvic floor and anal sphincters. The levator ani (lev ani) muscles blend with the puborectalis (pr) which in turn merges with the circular external sphincter (ext). The internal sphincter is continuous with the muscularis propria of the rectum, running the length of the anal canal and higher signal on STIR than the outer striated sphincter muscles. The intersphincteric space (yellow arrow) occupies the plane between the internal sphincter and the external sphincter muscles. The dentate line, though not visible radiologically, lies just below the puborectalis, around 2 cm from the anal verge (red broken line)
The anal canal undergoes epithelial transition from the glandular epithelium proximally to the squamous epithelium distally, divided by the dentate line. This lies approximately 2 cm from the anal verge or just below the level of the puborectalis muscle. The anal glands that produce mucus communicate with the anal lumen at this position. These can lie deep within the submucosa with some penetrating the internal sphincter to lie in the intersphincteric space.
Two sphincter muscles encircle the anal canal. The external sphincter is composed of striated muscle and is under voluntary control with three components – submucosal, superficial and deep – but it is not usually possible to separate these on MRI. The external sphincter is continuous proximally with the U- or V-shaped puborectalis muscle (Fig. 15.2) which itself merges with the levator ani muscles of the pelvic floor. The internal sphincter is composed of smooth muscle and represents the distal termination of the muscularis propria of the gut tube and is under autonomic control. The intersphincteric space refers to the plane between the external and internal sphincters and contains fat.
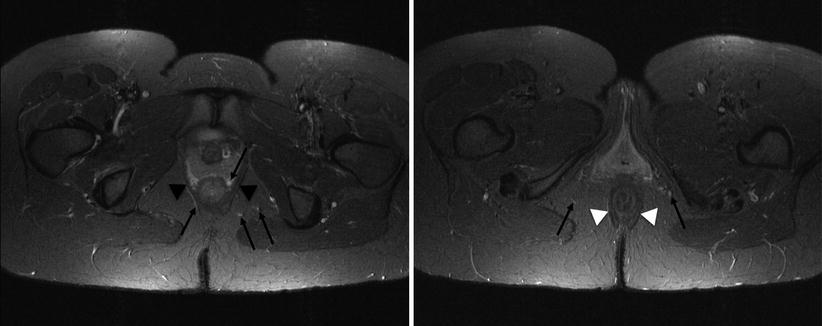

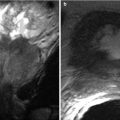
Fig. 15.2
The puborectalis wraps posteriorly around the lower rectum (black arrowheads), while the external sphincter encircles the lower rectum (white arrowheads). Potential pitfall: high signal vessels on STIR adjacent to the rectum and within the ischioanal fat (arrows) – these should not be mistaken for fistulas
The internal sphincter is responsible for 85 % of resting anal tone, but in most individuals it can be divided without causing a loss of continence. On the other hand while the external sphincter contributes only 15 % of resting anal tone, its strong voluntary contraction resists defecation, and division of the external sphincter can lead to incontinence.
The ischioanal fossa contains fat and fibroelastic connective tissue and is bordered by the levator ani muscles (superior), gluteus maximus (posterior), transverse perineal muscles (anterior), external anal sphincter and puborectalis (medial), obturator internus and the ischial tuberosity (lateral) and skin (inferior). High signal vessels within the ischioanal fat and intersphincteric plane should not be misdiagnosed as fistulas – a potential pitfall (Fig. 15.2).
Pathology and Evolution of Perianal Sepsis and Fistulas
In the first stages, a crypt abscess forms, usually in the intersphincteric plane, caused by obstruction of the mucous glands within crypts at the dentate line (sometimes called cryptoglandular sepsis). Patients often present with acute perianal pain with signs of local infection, and in some cases, the abscess will extend into the ischioanal fossa. These are typically dealt with surgically. In some cases the abscess will discharge to the perineum leaving a residual fistulous communication to the anal canal. Both the point of origin and the direction that the infection tracks (either between, through or above the external sphincter muscle) will decide the eventual course of the fistula.
It is important to differentiate the pathology of fistulas caused by glandular infection from other fistulating diseases. Crohn disease is precipitated by deep mucosal ulceration, which may arise either from the mucosa or the submucosal glands, while tumour-related ulceration typically results from necrosis of the tumour wall.
Technique for MR Fistulography
Technical Requirements for MRI
A 1.5 T (or 3 T) MRI scanner with external phased array surface coils is needed for adequate resolution and contrast. Endoanal coils are not recommended; although they provide very high-resolution imaging with excellent delineation of the sphincters, the limited field of view may not detect the extensions and abscesses that influence the surgical approach, and they are uncomfortable and invasive to patients [6].
Orientation, Field of View and Imaging Plane
Orientation of the scanning planes should be either parallel or perpendicular to the anal canal. Since the anal canal is approximately 45° from vertical, straight axial and coronal images fail to achieve correct alignment and distort the sphincter complex (Fig. 15.3). An adequate field of view should be selected to cover the whole perineum and extend above the levator muscles to include in inferior aspect of the presacral space for coverage of long fistula extensions, supralevator collections or osteomyelitis (Fig. 15.4). However, this requires balance: too large a field of view will diminish resolution.
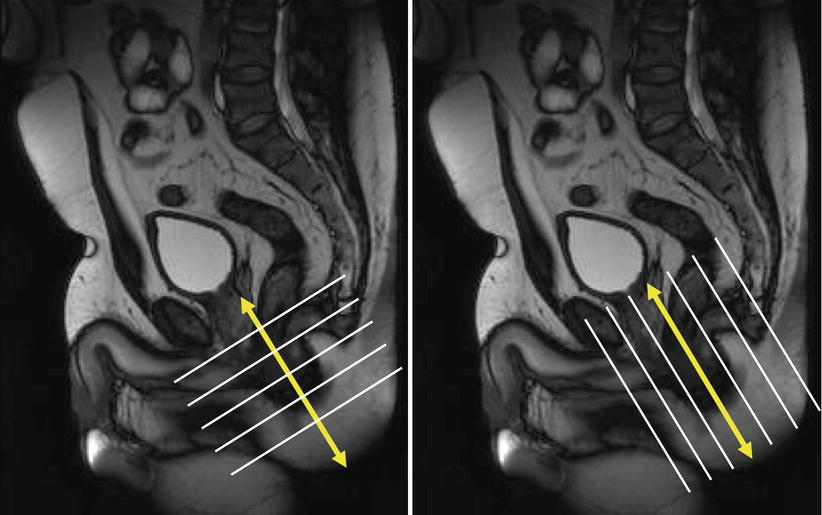
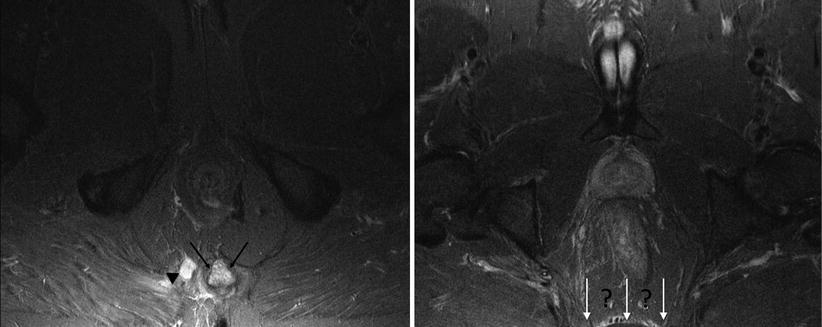

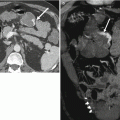
Fig. 15.3
Correct orientation to the anal canal (yellow arrows) – the axial and coronal planes should be orientated obliquely (white lines) and adequately cover the whole perineum

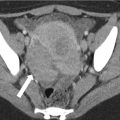
Fig. 15.4
Correct field of view is essential to assess disease extent. Patient with high signal in the coccyx (black arrows) due to osteomyelitis in fistulising Crohn disease (arrowhead). Poor technique results in inadequate coverage of the lower sacrum and presacral space (white arrows)
With respect to the imaging plane, an oblique axial plane is required to determine the relationship of the fistula to the sphincter mechanism and whether it traverses the external sphincter. It also best assesses horseshoe extensions and the radial location of the internal opening. The oblique coronal plane allows assessment of the true height of the internal opening within the canal and fistula relationship with the levator ani muscles (Fig. 15.5). Sagittal images are useful in selected cases, for example, in the assessment of anovaginal fistula or for posterior extension where sacral or coccygeal sepsis is suspected, but are not used routinely in the authors’ practice.
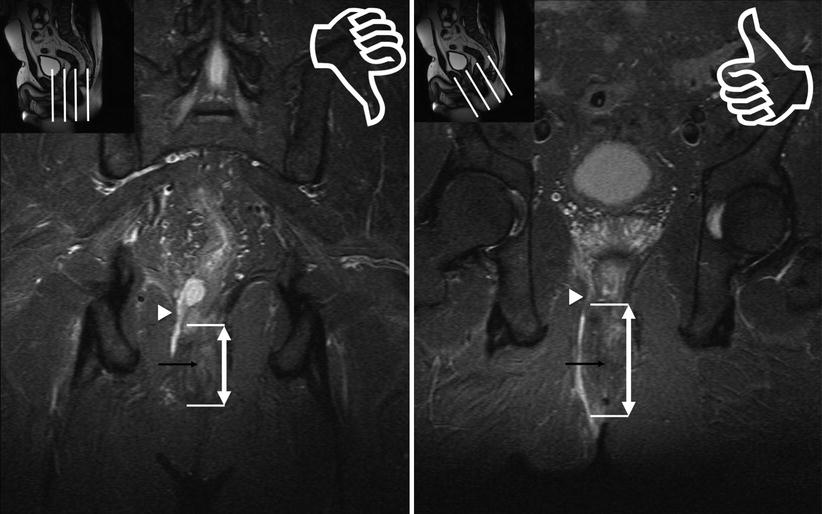

Fig. 15.5
Straight and oblique coronal images. Foreshortening effect with straight coronal (left). Correct oblique orientation to the plane of the anal canal (right) allows accurate estimation of the level of internal opening (black arrow) for this transsphincteric fistula with superior extension through the right levator in Crohn disease (arrowhead)
A slice thickness of 3–5 mm should be used to minimise the effect of volume averaging in detection of small fistulas or extensions – slices thicker than 5 mm should be avoided.
Sequence Selection and Rationale
Various sequence selections have been proposed for fistula imaging. Evidence for superiority of one sequence over another is lacking, and choice depends to a degree on personal preference. It is important that the sequences chosen provide anatomic precision to map the fistula course and determine its relationship with other structures [7].
In the authors’ experience, axial and sagittal gradient echo sequences (e.g. FISP) through the pelvis provide a general overview and exclude pelvic disease processes. They also facilitate orientation to the anal canal for more subsequent detailed ‘fistula-specific’ sequences.
In the detailed imaging of the fistula itself, there are three main sequence choices: STIR, T2 fat-suppressed (SPAIR/SPIR) and gadolinium-enhanced T1 with fat suppression (2D or 3D). A majority of UK radiologists use the STIR sequence for fistula assessment (Tolan, personal communication). Both STIR and fat-suppressed T2-weighted sequences provide good contrast between hyperintense fluid in the tract and the hypointense fibrous wall of the fistula, while enabling discrimination with the anal sphincter muscles [7]. Post-contrast fat-suppressed T1 sequences rely on increased enhancement of inflamed fistula tracks for visualisation against the normal enhancement of other structures (e.g. muscle) and non-enhancement of fat [8].
In our routine clinical practice, with a high throughput of patients with Crohn disease, we routinely perform both STIR and T1 fat-saturated post-gadolinium sequences in all cases to maximise detection of extensions and differentiate granulation tissue from pus within fistulas.
Gadolinium-Enhanced Sequences
T1 Fat-Saturated post-gadolinium (3D VIBE or 2D T1 FS)
Thin inflammatory tracks more conspicuous.
Inflammatory tissue bright, fibrotic areas intermediate signal.
Dark fluid/pus allows differentiation from bright enhancing inflammatory granulation tissue (not possible with STIR/T2 FS). Important in patients commencing immune modulator therapies (e.g. infliximab) to allow drainage of sepsis prior to treatment.
Enhancement within normal anatomical structures usually limited to the internal anal sphincter and small blood vessels
Pitfalls
Requires injection
Overcalling healing tracts or blood vessels as active fistulas
‘Water-Based’ Fistula Sequences
STIR or T2 with fat saturation (SPIR/SPAIR)
Relatively simple technique.
No injection required.
Provides high conspicuity for active fistula tracks.
Inflammatory tracks appear bright and fibrotic healed tracts appear dark.
Acquisition times can be long.
Pitfalls
Thin tracks or extensions can be difficult to demonstrate.
Inflammation and granulation tissue cannot be differentiated from pus.
Suggested MRI Fistula Protocol
FISP – 5 mm axial and sagittal (overview pelvis and orientation)
STIR – 3 or 4 mm oblique axial and coronal (plus sagittal for anogenital or supralevator fistula)
T1 FS post-gadolinium (3D VIBE) – 3 or 4 mm oblique axial and coronal (plus sagittal for anogenital or supralevator fistula)
Normal MRI Anatomy
The internal sphincter is normally more hyperintense on T2-weighted and STIR images than the external sphincter (Fig. 15.1) and shows enhancement on post-gadolinium T1 sequences. The intersphincteric space is a thin hypointense ring between the internal and external sphincters. The external anal sphincter is relatively hypointense on T2 FS and STIR sequences, with its lateral border contrasting against fat in the ischioanal fossa (on fat-saturated/fat-suppressed sequences) to a variable degree depending on the TR/TE selected (Figs. 15.1 and 15.2).
The anal canal is often shorter in females than males. In patients with prior sphincter injury for other reasons (e.g. obstetric injury, previous haemorrhoid or fistula surgery), a visible defect or area of thinning may be present in the sphincter muscles, which may be associated with low signal fibrosis from scarring.
Fistula Classification
Preoperative classification requires correct localisation of the fistula to the anal sphincter complex.
The two fistula types most commonly encountered on MR fistulography are intersphincteric and transsphincteric (Fig. 15.6) [7, 8]. Intersphincteric fistulas track along the intersphincteric plane inferiorly to the anal verge (Fig. 15.7). They are contained by the external sphincter throughout its course but may traverse the lowest fibres of the external sphincter, which interdigitate with the internal sphincter, to reach the skin.
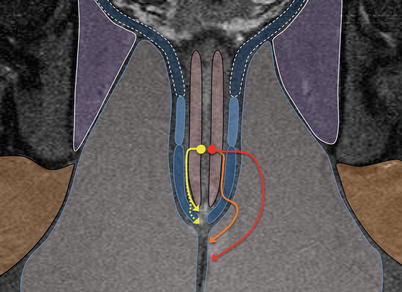
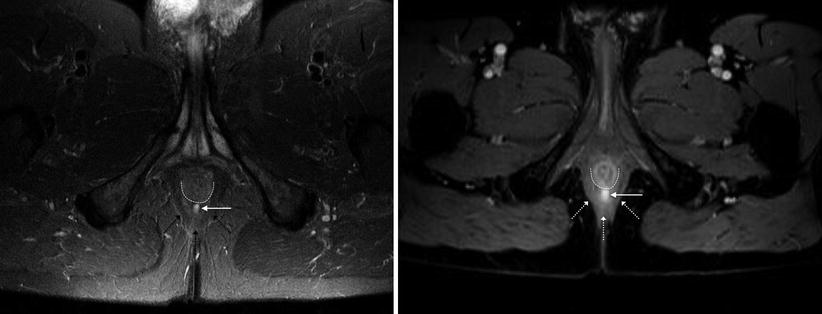

Fig. 15.6
Commonly encountered fistulas: yellow – intersphincteric fistula: confined to the intersphincteric plane. These may cross the lowermost fibres of the external sphincter (dotted arrow). Red and Orange – transsphincteric fistula: may traverse the external sphincter high (red) or pass some distance distally in the intersphincteric plane before crossing the external sphincter (orange)

Fig. 15.7
Intersphincteric fistula. STIR (left) and VIBE post contrast (right) demonstrate the high signal fistula (white arrow). The internal opening is at 6 o’clock at the level of the dentate line (lowest portion of the puborectalis – dotted arrows). The fistula runs outside the internal sphincter (dotted line) but does not cross the puborectalis muscle (dotted arrows)
Transsphincteric fistulas typically fall into two groups depending on the amount of external sphincter muscle that is crossed, and the fistula reaches the skin via the ischioanal fossa fat. ‘High’ transsphincteric fistulas traverse the external sphincter more proximally – in such cases surgical sphincterotomy may render a patient incontinent. ‘Low’ fistulas may track in the intersphincteric plane before crossing the external sphincter (Fig. 15.8) – in these cases fistulotomy sacrificing some of the sphincter mechanism is likely to preserve function. It is therefore important to provide the surgeon with an impression of the amount of external sphincter muscle involvement to inform surgical planning [8].
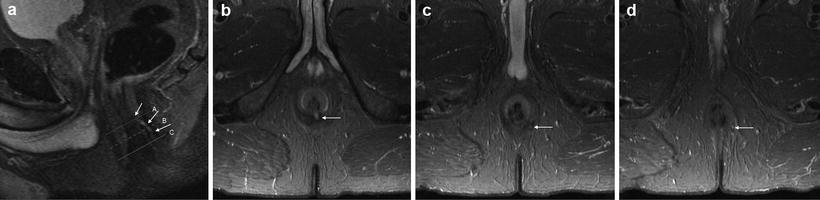

Fig. 15.8
Low transsphincteric fistula on STIR. Sagittal image demonstrates the fistula (arrows) opening in the mid canal at dentate line level at 6 o’clock (a), but the precise relationship to the external sphincter is unclear. Axial images confirm it passes through the lower external sphincter at 5 o’clock (b) before exiting to the perineum at 5’o’clock (c)
Less commonly observed fistulas are shown in Fig. 15.9. Superficial fistulas are typically identified and dealt with surgically without recourse to imaging, and so are not often encountered on MRI. They remain superficial to the internal sphincter, running submucosally to the anal verge (Fig. 15.10).
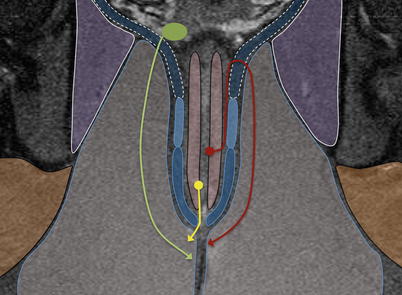
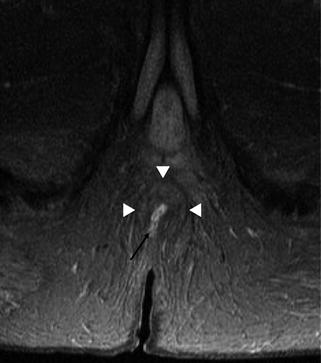

Fig. 15.9
Less common fistulas: yellow – superficial fistula: infection tracks in a submucosal plane to the anal verge. Maroon – supralevator fistula: sepsis tracks proximally in the intersphincteric plane before piercing the levator ani to descend through the ischioanal fossa. Green – extrasphincteric fistula: pelvic pathology generates sepsis, which tracks through the levator ani to the perineum

Fig. 15.10
Superficial fistula. The fistula (arrow) originates low in the anal canal to emerge at the anal verge without traversing the internal or external sphincter (arrowheads), by maintaining a submucosal plane
Supralevator fistulas are very rarely encountered but important to identify. In such cases, intersphincteric infection arising from the anal canal tracks superiorly before passing across the levator muscle and across the ischioanal fossa to the skin. If these are incorrectly classified by surgical exploration, this may result in unnecessary division of the anal sphincters or leave residual untreated fistula tracks after operation that will predispose for recurrence in the future.
Extrasphincteric fistulas arise in the pelvis (not the anal canal), most often in association with Crohn disease, tumour and pelvic sepsis. Infection superior to the levator muscle erodes through it to produce a fistula to the perineum for drainage (Fig. 15.11). The underlying cause should be treated where possible, which may require faecal diversion with a stoma to reduce contamination during healing [8].
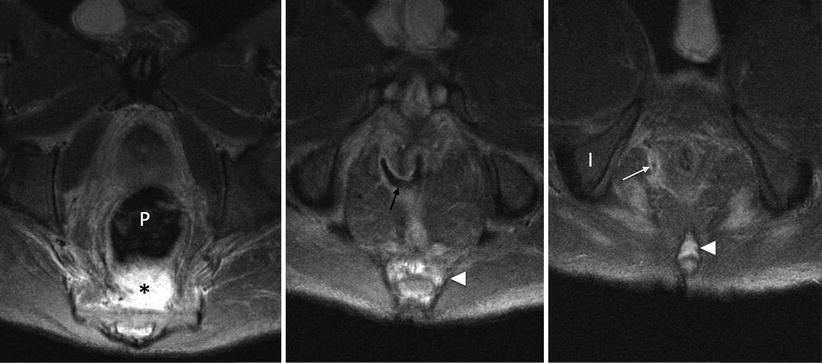

Fig. 15.11




4 weeks post reversal of loop ileostomy after ileal pouch anal anastomosis (IPAA). A presacral abscess lies behind the pouch (P) due to anastomotic breakdown. A defect at the anastomosis at 9 o’clock (black arrow) crosses the right levator and through the ischioanal fossa (white arrow) producing an extrasphincteric fistula. Note high signal in the coccyx and distal sacrum (white arrowhead) relative to the ischium (I) due to secondary osteomyelitis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree