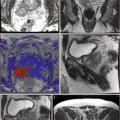
Fig. 11.1a ADC MRI
Fig. 11.1b DCE MRI
Fig. 11.1c T1-weighted MRI
Fig. 11.1d T2-weighted MRI
Fig. 11.1e Registered result
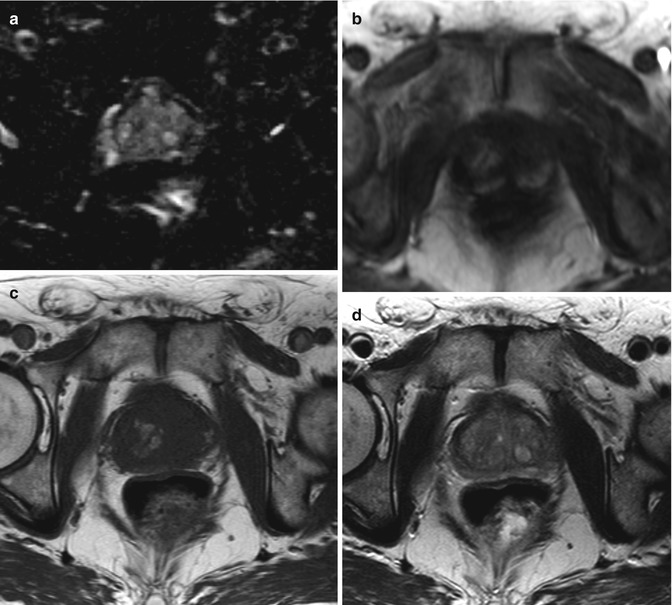
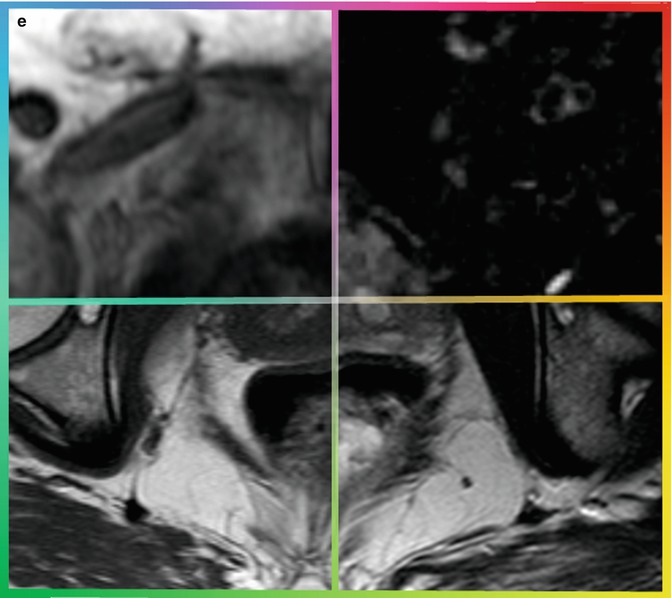
Fig. 11.1
Various MRI images (a–d) are acquired for a single patient. A computer registration algorithm brings these various images into spatial alignment (e), so orthogonal information from the various parameters can be used simultaneously to make an informed diagnosis
For multiparametric MRI, an accurate registration can allow the clinician to make a more informed diagnosis, but the result of image registration is not limited to diagnosis. The following sections discuss the current state-of-the-art computerized registration algorithms, specifically in how they relate to medical imagery.
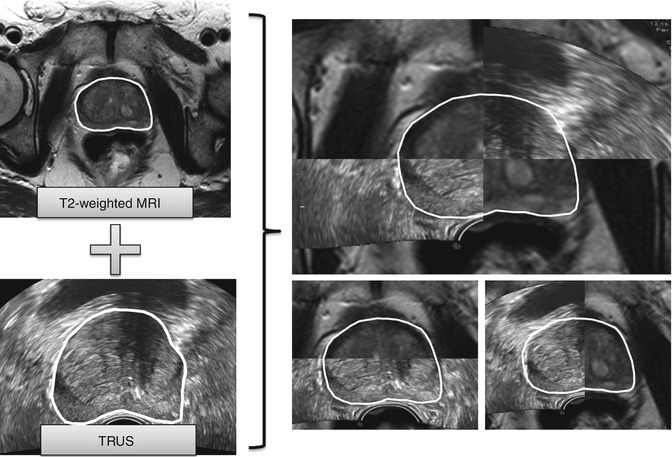

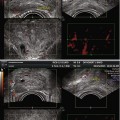
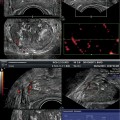
Fig. 11.2
A computerized registration algorithm brings a prostate MRI image in spatial alignment with a transrectal ultrasound (TRUS) image, which is necessary for guided biopsies
11.2 Clinical Advantages of MRI-Ultrasound Fusion Images
MRI offers significant diagnostic capabilities, and prostate cancer can frequently be detected using MRI (Turkbey and Choyke 2012). In addition, there is evidence that MRI can even be used to stage the aggressiveness of prostate cancer by being correlated with Gleason scores (Turkbey and Choyke 2012; Rastinehad et al. 2011; Rais-Bahrami et al. 2013).
However, when performing prostate biopsies for detecting cancer, an ultrasound image is utilized and is the current clinical standard due to its cost, ease of use, and widespread use by urologists. However, it is rife with problems such as low spatial resolution, ultrasound artifacts, and extremely low signal-to-noise ratio.
MRI-TRUS fusion devices aim to overcome these issues by utilizing MRI to target the lesion and incorporating this information with a TRUS biopsy to yield more precise biopsies (Pinto et al. 2011; Sonn et al. 2013; Natarajan et al. 2011; Zogal et al. 2011; Ukimura et al. 2012; Fiard et al. 2013; Marks et al. 2013). When performing a TRUS biopsy, a fusion system may wish to overlay the MRI information on top of the TRUS image, to help guide the biopsy. This requires that the MRI image be registered live with the TRUS image (Fig. 11.2).
Once the MRI and TRUS images are fused via image registration, a urologist can use the information derived from the MRI (such as the tumor outlined by an experienced radiologist) and guide the biopsy needle to that precise region within the prostate (Pinto et al. 2011; Sonn et al. 2013; Natarajan et al. 2011; Zogal et al. 2011; Ukimura et al. 2012; Fiard et al. 2013; Marks et al. 2013).
11.3 Devices That Offer MRI-TRUS Fusion
There are several examples of commercial systems for MRI-TRUS fusion and prostate biopsy guidance, some of which employ computerized registration algorithms, outlined below.
BiopSee ®: This device can only be used for transperineal biopsies. It has reported a guidance accuracy of 1.7 mm (Zogal et al. 2011), although explicit MRI-TRUS registration accuracy is not reported. The probe automatically rotates to the next target, but the needle guide requires manual adjustments.
GeoScan BioJet™: This system is relatively inexpensive, yet is also limited to transperineal biopsies and not compatible with many ultrasound systems. As of July 2013, the guidance and fusion accuracies are not reported and clinical validation is still required.
UC–Care Navigo™: This system is relatively inexpensive, although it is compatible with only a few ultrasound systems. While it is an image-guided biopsy system with the ability to integrate pathology lab findings, it currently offers no MRI fusion capabilities.
Biobot Mona Lisa: This is a robotic 3D prostate biopsy system with an accuracy guidance within 1.5 mm. However, it is limited to transperineal biopsies, offers no MRI fusion, and is currently awaiting regulatory approval.
Koelis Urostation: This is a software system which attaches to an existing ultrasound machine. It uses image registration to track, via computer algorithms, the location of the probe within the prostate. MRI fusion targeting accuracy was reported to be 84 % in phantoms (Ukimura et al. 2012). Initial feasibility studies (with 20 cases) show a 91 % sensitivity to significant cancer (Fiard et al. 2013), although systematic clinical validation is still required.
In vivo UroNav: This system from In Vivo/Philips offers MRI-TRUS fusion and has an easy to use freehand transducer for tracking. It was found to have approximately a 90 % detection rate for highly suspicious targets on MRI (Pinto et al. 2011). The system utilizes a rigid registration technique (no deformable registration, see Sect. 11.5) and is costlier than several other comparable systems, although it has been extensively clinically validated at the NCI.
Eigen Artemis: This system from Eigen was the first commercial MRI fusion-guided biopsy system. It utilizes both rigid and nonrigid registration algorithms (see Sect. 11.5) and uses a robotic arm to hold and maneuver the transducer to the target. It has validated in a number of clinical studies (Sonn et al. 2013; Natarajan et al. 2011; Marks et al. 2013) and is approximately two to three times more sensitive for detecting prostate cancer compared to traditional biopsies.
MIMviewer ®: This software package from MIM Software, Inc. offers TRUS-MRI fusion by plugging into existing ultrasound systems’ video feeds and performing software-based registration. It is significantly less costly than alternative devices since it does not require a new dedicated fusion ultrasound machine to be purchased.
11.4 Registration Overview
As stated previously, registration is the process of bringing multiple images into spatial alignment. The images are typically either 2D or 3D, although 4D time-series data is sometimes available. The goal of any registration algorithm is to determine a transformation which best aligns the images. This transformation can be as simple as translating the image (moving up/down or left/right) or as sophisticated as warping certain spots within the images (called “deformable registration”).
A computer algorithm essentially tries various transformations and finds the “optimal” one which best aligns the images. The workflow of a registration algorithm is illustrated in Fig. 11.3. There is always a reference “fixed” image which does not move, and a “moving” image, to which the transformations are applied. Various transformations are tried, and an “interpolator” generates a “moved” image for each of those transformations. The “moved” image which most closely matches the “fixed” image is chosen (via the use of an “optimizer”) and output. The following sections describe each of these processes in detail.
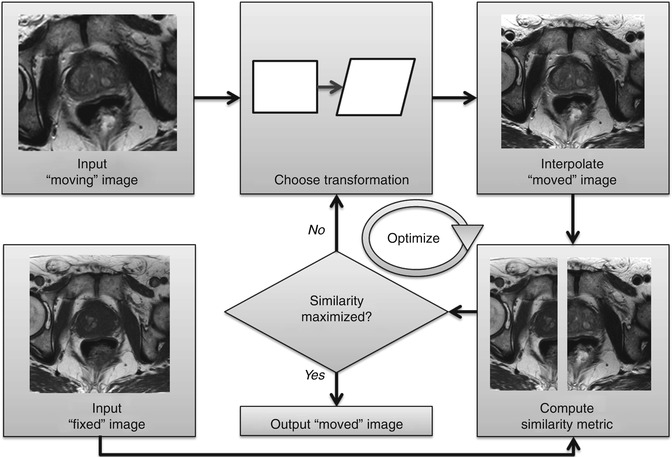

Fig. 11.3
Computerized registration workflow. A “moving” image is transformed until a similarity with a “fixed,” reference image is maximized
11.5 Transformations
A transformation is an integral component to any registration algorithm. It defines how to move, or deform, a given image. The two general categories of transformations are “linear” and “deformable” transformations.
A linear transformation is any translation, rotation, scaling, or shearing which is applied to the image as a whole. Figure 11.3 represents a linear transformation applied to a rectangle, creating a sheared rectangle. A transformation which only allows translation and rotation is considered “rigid.” When incorporating scaling and shearing, the transformation is considered “affine.” Rigid registration was used to fuse MRI and CT prostate images in Greene et al. (2009).
A deformable registration allows warping of an image at specific locations. Essentially, in its most general form, every single pixel (or tiny location within the image with an intensity value) is allowed to move anywhere else in the image. There are various types of deformable transformations including B-splines, thin plate splines, and finite element model transformations. Examples of algorithms which use splines to register prostate images can be found in Ogura and Mitra (Ogura et al. 2009; Mitra et al. 2012). Examples of algorithms which use finite element models to register prostate images can be found in Chi et al. (2006), Boubaker et al. (2009), Hensel et al. (2007), and Brock et al. (2008).
Whether using a linear or deformable registration, a series of numbers is used to represent the given transformation. In the case of linear transformations, these numbers may represent the amount to translate the image (in mm) or the magnitude of rotation (in degrees). In the case of deformable registration, these numbers may represent how certain regions of the image should warp relative to other regions.