Müllerian duct anomalies (MDAs) are a group of congenital uterine disorders that arise from arrest in development, incomplete fusion, or incomplete resorption of the mesonephric ducts. They are uncommon, usually asymptomatic, and are diagnosed incidentally at the time of delivery or during a routine gynecologic examination. Less commonly MDAs can cause infertility, endometriosis, recurrent miscarriages, and symptoms arising from an obstructed reproductive tract. The identification and classification of these anomalies are crucial to distinguish between those with a high risk for obstetric complications and that require surgical intervention from the less-threatening variants. It is also imperative to separate those anomalies that necessitate a transabdominal surgical procedure from those that can be treated with a hysteroscopic approach. The reproductive outcome and treatment options depend on the type of uterine malformation. Early identification of MDA can help prevent symptom development and obstetric complications.
This chapter will explain the controversial embryology of the MDAs and the currently used classification systems. The value of multiple imaging tests will be reviewed. Finally the imaging findings and management of different classes of MDAs will be discussed.
Definition
MDAs are a group of congenital uterine disorders that arise from arrest in development, incomplete fusion, or incomplete resorption of the mesonephric ducts.
Prevalence and Epidemiology
MDAs have a wide spectrum of clinical presentations, and their true incidence and prevalence rates are unclear. A critical appraisal of pooled studies examining the prevalence of uterine anomalies suggests that women with recent miscarriages have higher prevalence of MDAs (13% to 17%) compared with the general fertile population (6.7%) and infertile population (7.3%). Septate uterus is the most common anomaly in the infertile population. Arcuate uterus, usually considered a normal anatomic variant, is present in 12.2% of women suffering from repeated miscarriages and is 3 times more prevalent in this population compared with the general fertile population (3.8%).
Embryologic Classification
In the female embryo, paired paramesonephric (müllerian) ducts develop into the reproductive system through three stages that include organogenesis, fusion, and septal resorption.
At the sixth week of embryogenesis, male and female embryos are indistinguishable, and both have paired paramesonephric (müllerian) and mesonephric (wolffian) ducts. Failure in formation of one or both müllerian ducts results in uterine agenesis, hypoplasia, or unicornuate uterus.
As the embryo grows, the mesonephric and paramesonephric ducts grow parallel to each other and toward the urogenital sinus. The lower parts of müllerian ducts are derived from or induced by the mesonephric ducts. At week 9 and before reaching the urogenital sinus, the caudal part of müllerian ducts join together and fuse to form a single lumen of uterovaginal canal, which later develops into the uterine corpus, cervix, and the upper four fifths of the vagina. The upper parts of müllerian ducts, which are derived from coelomic epithelium, remain unfused and develop into fallopian tubes. Defects in fusion will result in bicornuate or didelphys uterus. The mesonephric ducts regress in the female embryo secondary to absence of a factor associated with the Y chromosome.
Simultaneously sinovaginal bulbs arise from the urogenital sinus and grow to fuse with the distal uterovaginal canal, where a cellular mass called vaginal plate is formed (vertical fusion). The vaginal plate elongates and canalizes during the second trimester to make the lowest one fifth of the vagina. Thus the vagina has a dual origin: müllerian ducts contribute to the upper four fifths of the vagina (müllerian vagina), and the lower one fifth develops from the sinovaginal bulbs. Recent genetic and molecular studies question the validity of this embryologic model and suggest a müllerian origin for the entire vagina.
At the twelfth week of fetal development, myometrium has developed and the uterus has gained its normal appearance. Meanwhile septal resorption begins, and the septum between the fused müllerian ducts regresses to make a single uterine cavity. Failure of this resorption results in a septate or arcuate uterus.
The hymen is the interface of the elongated vaginal plate with the urogenital sinus. Its origin is thought to be a posterior invagination of the urogenital sinus. It will usually perforate by birth.
The traditional embryologic hypothesis suggests that müllerian ducts’ fusion begins at the müllerian tubercle and proceeds unidirectionally in a cranial to caudal direction, like a zipper, and the resulting septum regresses bidirectionally shortly after at any point of fusion.
Classification
A classification was proposed by Buttram and Gibbons in 1979 and was revised by the American Society for Reproductive Medicine (ASRM; formerly known as American Fertility Society) in 1988 ( Figure 18-1 ).
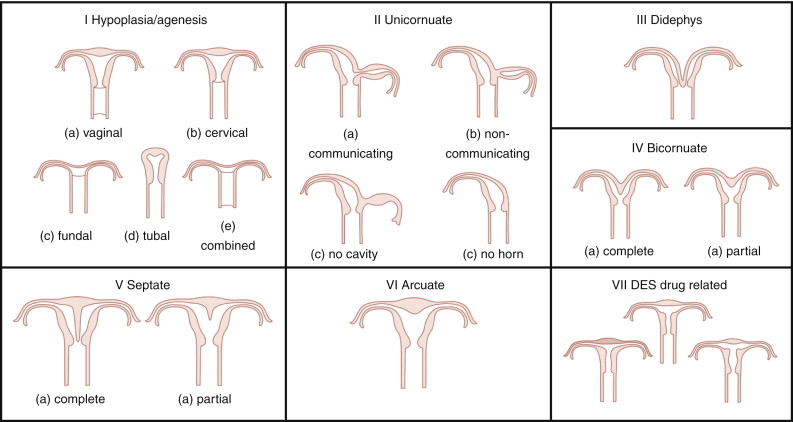
Categories I and II of this classification include anomalies where there is developmental failure of one or both müllerian ducts. Categories III and IV include anomalies in which there is some degree of failure in fusion of the ducts. Categories V and VI comprise anomalies in which there is failure in resorption of the septum.
The ASRM classification has some limitations: It is subjective and does not specify the diagnostic criteria for each type of anomaly, and some rare müllerian anomalies such as the presence of a duplicated cervix or vagina with normal uterus cannot be explained by the traditional müllerian fusion theory. An alternative hypothesis proposed by Muller et al. in 1967 suggests bidirectional fusion of müllerian ducts where fusion begins at the level of the uterine isthmus and continues in both cephalad and caudal directions. The resorption of the septum is also bidirectional and begins at the isthmus, with unification first of the cervix and vagina followed by unification of the uterine cavity. Anomalies such as duplicated cervix and vagina with normal uterus are explained with failure of fusion of the müllerian ducts below the uterine isthmus with normal fusion and resorption of the septum above it. The ASRM describes primarily uterine abnormalities, whereas anomalies of the vagina, adnexa (aplasia/hypoplasia), and associated renal or skeletal malformations are not addressed.
In 2005, Oppelt et al. suggested an alternative classification with the acronym VCUAM. In this classification, female external and internal genital organs are divided according to the anatomy. The VCUAM classification subdivides the female genital organs as follows: v agina, c ervix, u terus, a dnexa, and associated m alformations, making description of complex anomalies easier.
A third classification proposed by Acien et al. in 2004 is based on embryologic origin of the organs of the genitourinary tract. This classification, however, is complex and is rarely used in clinical practice.
Imaging Techniques
Radiology
Hysterosalpingography
The sensitivity and specificity of hysterosalpingography (HSG) for uterine malformations are approximately 44.4% and 96.4%, respectively. HSG is the first imaging modality in the assessment for infertility and is primarily used to determine tubal patency. It is accurate in the diagnosis of diethylstilbestrol (DES)-exposed uterine anomalies. Because the external uterine contour cannot be evaluated, however, HSG is insufficient to differentiate between the other classes of MDAs with accuracy, particularly the bicornuate and septate uterus. These anomalies have similarly appearing uterine cavities but require different surgical management. Measurement of the angle between the uterine horns by HSG is not accurate for differentiation of bicornuate from septate uterus.
Ultrasound
Two-Dimensional Transvaginal Ultrasound
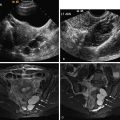
Pooled data comparing two-dimensional (2D) ultrasound (US) and hysteroscopy suggest a low sensitivity of less than 60% and high specificity of nearly 100% for detection of uterine anomalies. The wide availability makes this modality a useful screening tool along with HSG. Both HSG and 2D US, however, have the lowest accuracy and are not preferred modalities for diagnosis and differentiation of müllerian anomalies. The most important limitation for diagnosis of MDAs with 2D US is inadequate fundal visualization.
Sonohysterography
Sonohysterography is performed with saline infusion and offers improved delineation of the endometrium and uterine morphology. This modality detects and classifies uterine malformations with a higher accuracy than HSG or 2D US alone.
Three-Dimensional Ultrasound
Three-dimensional (3D) US has higher specificity and reproducibility than the conventional 2D US in detecting uterine anomalies. Unlike 2D US, 3D US can show the fundal surface of the uterus in the coronal plane, enabling the user to distinguish arcuate, septate, and bicornuate uteri. Using special software, a combination of surface-rendered and multiplanar images allows improved detection and characterization of anomalies. Reported sensitivity, specificity, positive predictive value, and negative predictive value for 3D US in detection of müllerian anomalies approach 100% compared with hysteroscopy or combined hysteroscopy–laparoscopy. It has been suggested that 3D US should become the initial modality to evaluate patients with recurrent miscarriages.
The advantages of 3D US include ease of use, automatic generation of 3D volume-rendered images, and less operator dependence. Three-dimensional US should be performed in the luteal phase because the endometrium is hyperechoic and easily identified. Limitations of 3D US include image distortion from acoustic shadowing of uterine myomas, nonvisualization of fallopian tubes, and inability to determine thickness and length of vaginal septum.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is currently the accepted standard in imaging evaluation of müllerian anomalies. MRI is nearly 100% accurate in the identification and characterization of MDAs. For müllerian anomalies, MRI diagnosis has a reported accuracy of 95% based on surgical confirmation.
The external and internal anatomy of the uterus, as well as the outer contour of the uterine fundus, is visualized using the multiplanar imaging capability of MRI. In addition, evaluation of the kidneys can be performed to identify commonly associated malformations.
T2-weighted imaging in the axial, sagittal, and coronal planes is the preferred technique to study the uterine body. The axial plane, centered on the long axis of the uterus, often yields the most information, and a T2-weighted sequence with high spatial resolution should be performed. Endometriosis and obstruction anomalies such as hematosalpinx or hematocolpos are better evaluated on combined T1- and T2-weighted images. To diagnose complex vaginal anomalies such as vaginal duplication or vaginal septum, the vagina may be distended with an inert material such as US gel before imaging.
The uterine septum may have myometrial (superior portion) and fibrous (inferior portion) components, which usually can be assessed in the axial plane.
A unicornuate uterus with cavitary noncommunicating rudimentary horn and a didelphys uterus with transverse vaginal septum are two anomalies that cause obstruction and hematometra. Didelphys uterus is the only anomaly that causes hematocolpos. MRI differentiates these and other complex anomalies with high accuracy.
MRI allows better visualization of pelvic anatomy and associated renal anomalies than 3D US; however, 3D US is less expensive and provides high spatial resolution of small anatomic structures.
Classification of MÜllerian Duct Anomalies
Class I: Hypoplasia/Agenesis
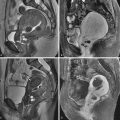
Failure of müllerian duct development results in uterovaginal agenesis or hypoplasia, which accounts for approximately 5% to 10% of all müllerian anomalies. The cervix may be absent or distorted in this class of anomalies. The proximal vagina is either absent or hypoplastic. The most common presentation is Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome, which presents with vaginal agenesis from the introitus, and uterine abnormalities that range from an absent uterus (90% of cases) to rudimentary uterine remnants (10% of cases). This syndrome is the second most common cause of primary amenorrhea after gonadal dysgenesis. Patients have normal female karyotype with normal ovaries and fallopian tubes. The secondary sexual characteristics and external genitalia are normal except that a shallow vaginal pouch may be present representing the urogenital sinus origin of the distal vagina. In uterine hypoplasia the presence of a functioning endometrium in an obstructive setting results in cyclic abdominal pain during menstrual periods and hematometra.
Anomalies of the upper urinary tract are present in 30% to 40% of cases, with renal agenesis and ectopic pelvic kidney being the most common variants.
Diagnosis
Clinical diagnosis in patients with primary amenorrhea is made after ruling out hormonal and karyotype abnormalities and an imperforate hymen and is confirmed by radiologic or laparoscopic investigations. HSG has no role in diagnosis. Two-dimensional US can identify an abnormal uterus with normal ovaries and associated renal anomalies. Three-dimensional US is more sensitive for evaluation of the small uterine remnant. MRI can differentiate between uterine agenesis ( Figure 18-2 ) and hypoplasia on sagittal images, although small remnants may be missed. Uterine hypoplasia is seen as a small hypointense uterine remnant on T2-weighted images with poorly differentiated zonal anatomy ( Figure 18-3 ). Vaginal anomalies are best seen on axial planes when no normal vaginal tissue is identified between the rectum and the urethra. The correct diagnosis of MRKH syndrome depends on visualization of normal ovaries on MRI. Associated renal anomalies are identified on coronal images.


Management
These types of anomalies have no reproductive potential. The goal of treatment is to restore normal sexual function with the creation of a neovagina, accomplished by vaginal dilation or surgical reconstruction.
Class II: Unicornuate Uterus
Unicornuate uterus results from partial or complete lack of development of one müllerian duct and accounts for 20% of all MDAs.
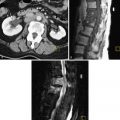
There may be isolated agenesis of one horn, but most frequently there is a rudimentary horn (65%), which may be noncavitary (33%) or associated with endometrium (cavitary) (32%). Cavitary rudimentary horn is further subdivided into two groups, namely, communicating, if the endometrial cavity communicates with contralateral cavity (10%), and noncommunicating, when the endometrium is isolated within the rudimentary horn (22%). There is a single normal cervix.
A simple unicornuate uterus or a unicornuate uterus with a communicating rudimentary horn may be an incidental finding during an infertility workup. Alternatively a patient with a cavitary noncommunicating rudimentary horn may present with cyclic abdominal pain during menstrual periods. These women are at risk for a rare and dangerous form of ectopic pregnancy, attributable to transperitoneal migration of sperm. Fertilization of an egg and implantation in the rudimentary horn can result in a fetus of 14 to 16 weeks before the rudimentary horn ruptures, with massive hemorrhage and exsanguination. Therefore the discovery of a rudimentary horn is usually followed by its surgical removal.
The reproductive performance of women with a unicornuate uterus is poor, with pooled data suggesting miscarriage rate of 34%, preterm delivery of 20%, intrauterine fetal demise of 10%, and 50% live birth rate. Diminished uterine muscle mass, abnormal uterine blood flow, and cervical incompetence account for the adverse obstetric outcomes. Gynecologic complications include endometriosis, most common with noncommunicating cavitary rudimentary horns. The highest rate of associated upper urinary tract anomalies is seen in this class (40%). Renal agenesis, the most frequent anomaly, is contralateral to the unicornuate horn.
Diagnosis
HSG usually demonstrates a single laterally deviated uterine canal or two asymmetric canals ( Figure 18-4 ). Noncommunicating rudimentary horn is missed with this modality. Two-dimensional US is not an accurate modality to diagnose unicornuate uterus; the conventional sagittal and transverse views often fail to identify the simple unicornuate uterus and may detect it as a normal uterus. Furthermore, the noncavitary rudimentary horn is not visualized or misdiagnosed as a uterine or adnexal mass. The thin endometrial lining of the cavitary rudimentary horn may not be identified.