Abstract
Multifocal ill-defined opacities most often result from multiple consolidations but must be distinguished from invasive or hemorrhagic tumors. This is not a common appearance for community-acquired pneumonia, but when it occurs this appearance indicates a serious infection that is likely caused by a virulent organism. Patients with a documented viral infection such as influenza who develop this pattern have most likely developed a superimposed bacterial pneumonia. Multifocal air space opacities are a common appearance for hospital-acquired pneumonias, especially for patients in the intensive care setting. Fungal pneumonias should be considered when the chest x-ray is suggestive of pneumonia and cultures for bacterial infection are negative. Immune compromised patients are at high risk for aggressive fungal infections. Multifocal air space opacities are not a common appearance for tuberculosis, but it must be excluded in patients who either present with associated cavities or develop cavities. Invasive mucinous adenocarcinoma is the most likely tumor to have this appearance and must be considered in an afebrile patient with a chest x-ray that looks like a multifocal pneumonia. This is a rare appearance for chronic lung diseases, but it may result from sarcoidosis, cryptogenic organizing pneumonia, hypersensitivity pneumonitis, and silicosis or coal worker’s pneumoconiosis.
Keywords
actinomycosis, aspergillosis, blastomycosis, candidiases, coal worker’s pneumoconiosis, coccidioidomycosis, cryptococcosis, cryptogenic organizing pneumonia, Escherichia coli , Haemophilus influenzae , histoplasmosis, hypersensitivity pneumonitis, invasive mucinous adenocarcinoma, Klebsiella , Legionella , lymphoma, mucormycosis, Nocardia , Pseudomonas , sarcoidosis, silicosis, sporotrichosis, Staphylococcus , Streptococcus
Questions
- 1.
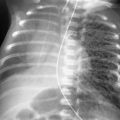
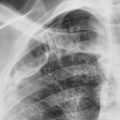
Which one of the following is the most likely diagnosis in the case seen in Fig. 16.1 ?
- a.
Tuberculosis.
- b.
Lung cancer
- c.
Melanoma.
- d.
Silicosis.
- e.
Pneumonia.

Fig. 16.1
- a.
- 2.
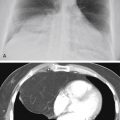
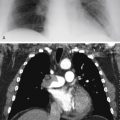
Referring to Fig. 16.2 , the combination of hilar adenopathy and multifocal ill-defined opacities is most consistent with which one of the following?
- a.
Granulomatosis with polyangiitis.
- b.
Sarcoidosis.
- c.
Hypersensitivity pneumonitis.
- d.
Langerhans cell histiocytosis.
- e.
Choriocarcinoma.

Fig. 16.2
- a.
- 3.
The presence of an air bronchogram throughout a large irregular opacity is inconsistent with which one of the following diagnoses?
- a.
Silicosis.
- b.
Invasive mucinous adenocarcinoma.
- c.
Lymphoma.
- d.
Granulomatosis with polyangiitis.
- e.
Sarcoidosis.
- a.
Discussion
Multifocal ill-defined opacities (see Fig 16.1 ) result from a great variety of diffuse pulmonary diseases ( Chart 16.1 ). This pattern is sometimes referred to as a patchy alveolar pattern, but it should be contrasted with the bilaterally symmetric, diffuse, coalescing opacities described as the classic appearance of air space disease in Chapter 15 . Many of the entities that cause multifocal ill-defined opacities do result in air space filling, but they also may involve the bronchovascular and septal interstitium. Acute diseases may present as patchy scattered opacities and progress to complete diffuse air space consolidation. Some of the additional signs of air space disease are also encountered in this pattern, including air bronchograms, air alveolograms, and a tendency to be labile.
- I.
Infectious diseases
- A.
Bacterial pneumonias ( Staphylococcus, Streptococcus, Pseudomonas, Legionella, 498 Klebsiella, Haemophilus influenzae, 428 Escherichia coli , other gram-negative bacteria, Nocardia 29 , 145 )
- B.
Fungal pneumonias (histoplasmosis, 97 blastomycosis, 450 candidiases, 56 actinomycosis, 26 , 91 coccidioidomycosis, aspergillosis, 591 cryptococcosis 229 , 346 , 499 mucormycosis, 33 sporotrichosis 89 )
- C.
- D.
Viral 95 , 382 , 599 and mycoplasma pneumonias 170 , 250
- E.
Rocky Mountain spotted fever 333 , 365
- F.
Pneumocystis jiroveci pneumonia
- G.
Paragonimiasis 416
- H.
Q fever 385
- I.
Atypical mycobacteria in patients with acquired immunodeficiency syndrome (AIDS) 363
- J.
Severe acute respiratory syndrome (SARS) 412
- K.
- A.
- II.
Autoimmune diseases
- A.
Sarcoidosis 460
- B.
Granulomatosis with polyangiitis 4 , 177
- C.
Goodpasture syndrome 441
- D.
Connective tissue diseases (e.g., rheumatoid arthritis, scleroderma, dermatomyositis) complicated by diffuse alveolar damage (DAD)
- E.
Systemic lupus erythematosus 441 (lupus pneumonitis or hemorrhage)
- A.
- III.
Neoplasms
- A.
Invasive mucinous adenocarcinoma (formerly classified as bronchioloalveolar cell carcinoma) 9 , 457 , 597 , 598
- B.
Metastases (e.g., vascular tumors, malignant hemangiomas, choriocarcinoma, 341 adenocarcinoma 526 )
- C.
- A.
- IV.
Lymphoproliferative disorders
- A.
Non-Hodgkin lymphoma 21 , 27 (mucosa-associated lymphoid tissue [MALT] lymphoma is most common) 543
- B.
Hodgkin lymphoma (rarely primary in lung)
- C.
Lymphomatoid granulomatosis 343 , 345 , 543
- D.
Posttransplant lymphoproliferative disorder 119
- E.
- F.
- A.
- V.
Environmental diseases
- A.
Hypersensitivity pneumonitis (allergic alveolitis) 86 , 299 , 565 , 570
- B.
Coal worker’s pneumoconiosis
- C.
Silicosis
- A.
- VI.
Smoking-related diseases
- VII.
Idiopathic
- A.
- B.
Acute interstitial pneumonitis (AIP) 12
- C.
Cryptogenic organizing pneumonia (COP) 86 , 329
- D.
Eosinophilic pneumonitis 85 , 181 , 344 (idiopathic, drug reaction, 489 or secondary to parasites 175 )
- A.
- VIII.
Other disorders
- A.
- B.
Radiation pneumonitis 384 , 425
- C.
Metastatic pulmonary calcification (secondary to hypercalcemia) 158 , 228
- D.
Fat emboli
- A.
Because many of the entities considered in the differential are in fact primarily interstitial diseases, complete examination of the chest radiograph may reveal an underlying fine nodular or reticular pattern. Distinction of this multifocal pattern from the fine nodular pattern may also become somewhat of a problem because the definition of the opacities is one of the primary distinguishing characteristics of the two patterns. The description for miliary nodules usually requires that the opacities be sharply defined, in contrast to the less defined opacities currently under consideration. Most entities considered in this differential produce opacities that are larger than 1 to 2 cm in diameter, in contrast to the fine nodular pattern in which the opacities tend to be less than 5 mm in diameter. Additionally, this pattern may result from diseases that cause multiple larger nodules and masses. Some tumors may be locally invasive and appear ill defined because of their growth pattern, whereas others may develop complications such as hemorrhage. Because the differential for multifocal ill-defined opacities is lengthy, its identification obligates the radiologist to review available serial examinations, carefully evaluate the clinical background of the patient, and recommend additional procedures.
Infectious Diseases
Bacterial Bronchopneumonia
Multiple areas of consolidation are the characteristic pattern of bacterial bronchopneumonia (see Fig 16.1 ; answer to question 1 is e ). Because this type of infection spreads via the tracheobronchial tree, large areas of air space consolidation are often preceded by lobular opacities 238 that tend to be ill defined because the fluid and inflammatory exudate that produce the opacities spread through the interstitial planes in addition to spilling into the alveolar spaces. 235 In some cases, the lobular pattern may have sharply defined borders where the exudate abuts an interlobular septum. The size of the radiologic opacities depends on the number of contiguous lobules involved. Intervening normal lobules lead to a very heterogeneous appearance. As the infection progresses, the consolidations will begin to coalesce and form a pattern of multilobar consolidation ( Fig 16.3 ) that may even become indistinguishable from the diffuse confluent pattern commonly associated with alveolar edema. This is a common pattern for hospital-acquired pneumonias.

The radiologic patterns of bronchopneumonia are determined by the virulence of the organism and the host’s defenses. The primary sites of injury are the terminal and respiratory bronchioles. The disease starts as an acute bronchitis and bronchiolitis. The large bronchi undergo epithelial destruction and infiltration of their walls by polymorphonuclear leukocytes. Epithelial destruction results in ulcerations that are covered with a fibrinopurulent membrane and that contain large quantities of multiplying organisms. As the inflammatory reaction spreads through the walls of the bronchioles to involve the alveolar walls, there is an exudation of fluid and inflammatory cells into the acinus, which results in the pattern of multifocal consolidations. As noted in Chapter 15 , patients with bronchopneumonia occasionally have only one lobe predominantly involved, but there are almost always other areas of involvement. An unusually virulent organism or failure of the patient’s immune response leads to rapid enlargement of the multifocal opacities and, finally, to diffuse air space consolidations. This has been observed in patients infected by common organisms and those infected with unusual organisms, including those with Legionnaires’ disease and those infected by Legionella micdadei (Pittsburgh pneumonia agent). 311 , 436 With more aggressive organisms, such as Staphylococcus aureus and Pseudomonas , a necrotic bronchitis and bronchiolitis lead to thrombosis of lobular branches of the small pulmonary arteries, which accounts for the cavitation seen in necrotizing pneumonias such as methicillin-resistant Staphylococcus aureus (MRSA; see Chapter 24 ).
Septic Emboli
Septic emboli are another source of severe pulmonary infection that occurs when a bolus of infectious organisms are released into the blood and spread to the peripheral pulmonary vessels. The first radiologic signs of septic emboli are small, ill-defined peripheral opacities. As the infection spreads, the size of the opacities increases, leading to multifocal peripheral opacities. Septic embolization also has a high probability of cavitation, which makes it different from many of the other causes of this pattern. Clinical correlation is essential in the diagnosis of septic embolization. There should be a history of a significant febrile illness. Risk factors for septic embolism include sepsis, osteomyelitis, cellulitis, carbuncles, and right-sided endocarditis. Patients with right-sided endocarditis frequently have a history of intravenous drug abuse and are at increased risk for MRSA infection.
Viral Pneumonia
Viruses produce their effect in the epithelial cells of the respiratory tract, leading to tracheitis, bronchitis, and bronchiolitis. 295 The bronchial and bronchiolar walls become very edematous, congested, and infiltrated with lymphocytes. The bronchial infiltrate may extend into the surrounding peribronchial tissues, which become swollen. This infiltrate spreads into the septal tissues of the lung, leading to a diffuse, interstitial, mononuclear, cellular infiltrate. The changes in the airways may extend to the alveolar ducts, but most severe changes occur proximal to the terminal bronchioles. The adjacent alveolar cells, both type 1 and type 2, become swollen and detached. These surfaces then become covered with hyaline membranes. In fulminant cases, there are additional changes in the alveoli. The alveoli are filled with a mixture of blood, edema, fibrin, and macrophages. In the most severely affected areas, there is focal necrosis of the alveolar walls and thrombosis of the alveolar capillaries, leading to necrosis and hemorrhage.
The radiologic pattern of a viral pneumonia depends on both the virulence of the organism and host defenses. 50 The mildest cases of viral infection are confined to the upper airways and manifest no radiologic abnormality. The earliest radiologic abnormalities are the signs of bronchitis and bronchiolitis, which may include peribronchial thickening and signs of air trapping. When the infection spreads into the septal tissues, a reticular pattern with interlobular septal lines may result, as described in Chapter 18 . The more serious cases lead to hemorrhagic edema and areas of air space consolidation. These opacities may be small and appear as a fine nodular pattern similar to that described in Chapter 17 , but the nodules tend to be less well defined than the classic miliary pattern. As the process spreads, lobular consolidations develop, as in bacterial bronchopneumonia. In the most severe cases, the lobular consolidations may coalesce into diffuse consolidation, resembling pulmonary edema or bacterial bronchopneumonia.
Influenza viral pneumonia should be suspected in patients with typical influenza symptoms of fever, dry cough, headache, myalgia, and prostration. As the infection spreads to the lower respiratory tract, the patient notices an increased production of sputum that may be associated with dyspnea, pleuritic chest pain, or both. Physical examination reveals rales that may be accompanied by diminished or harsh breath sounds. Because of the bronchial involvement, wheezes are occasionally noted. High-resolution computed tomography (HRCT) may reveal multifocal ground-glass opacities while the chest radiograph appears to be normal. The chest radiographic patterns evolve from a minimal reticular pattern to small, ill-defined nodules to lobular consolidations, and finally to diffuse confluent opacities. Influenza pneumonia may be complicated by adult respiratory distress syndrome or superimposed bacterial pneumonia. At this time, laboratory examination of the sputum and blood becomes paramount to rule out a superimposed bacterial bronchopneumonia. The organisms most likely to present a superimposed bacterial infection are pneumococci, staphylococci, streptococci, and H. influenzae. In this clinical setting, the development of a superimposed cavity virtually confirms a superimposed bacterial infection. Other causes of viral pneumonia are less commonly encountered in patients with normal immunity, but have been reported with hanta viruses, Epstein-Barr virus, adenoviruses, 295 and SARS. 412
Fulminating cases of viral pneumonia are rarely encountered in patients with normal immunity but may occur in patients with suppressed immune systems including those receiving high-dose steroid therapy, patients receiving chemotherapy, patients with AIDS, organ transplant patients, pregnant women, and older adults. Herpes simplex, varicella, rubeola, cytomegaloviruses, and adenoviruses occur mainly in immunocompromised patients. 295
Varicella pneumonia characteristically occurs from 2 to 5 days after the onset of the typical rash. It is most commonly noted in infants, pregnant women, and adults with altered immunity. Approximately 10% of adult patients may have some degree of pulmonary involvement. In contrast to what is seen in bacterial pneumonias, sputum examination shows a predominance of mononuclear cells and giant cells. As in the consideration of other viral pneumonias, the possibility of superimposed bacterial infection is best excluded by laboratory examination of the sputum and blood. Because the viruses of herpes zoster and varicella are identical, patients with an atypical herpetic syndrome consisting of a rash with pain that follows the nerves of a single dermatome are also at increased risk for development of this type of pneumonia.
Rubeola (measles) pneumonia may be more difficult to diagnose by clinical criteria because the pneumonia occasionally precedes the development of the rash. As with other viral pneumonias, the clinical findings are nonspecific and consist of fever, cough, dyspnea, and minimal sputum production. Increasing sputum production requires exclusion of a superimposed bacterial pneumonia. Timing is important for evaluating a pneumonia associated with measles. The development of primary viral pneumonia in rubeola is synchronous with the first appearance of a rash, whereas secondary bacterial pneumonias are most likely to occur from 1 to 7 days after the onset of the rash. Bacterial infection is strongly suggested in the patient with a typical measles rash whose condition improves over a period of days before pneumonia develops. A third type of pneumonia associated with measles pneumonia is histologically referred to as giant cell pneumonia. This may follow overt measles in otherwise normal healthy children, but children with an altered immune system may develop subacute or chronic, but often fatal, pneumonias. Pathologically, giant cell pneumonia is characterized by an interstitial mononuclear infiltrate with giant cells.
Cytomegalic inclusion disease 2 has few distinguishing clinical features, has a radiologic presentation suggestive of bronchopneumonia, and is frequently fatal in patients who are immunosuppressed. Histologically, the lung reveals a diffuse mononuclear interstitial pneumonia accompanied by considerable edema in the alveolar walls that may even spill into the alveolar spaces. In addition, the alveolar cells have characteristic intranuclear and intracytoplasmic occlusions. The radiologic opacities are the result of cellular infiltrates in the interstitium, as well as intra-alveolar hemorrhage and edema. Other viruses, including the coxsackie viruses, parainfluenza viruses, adenoviruses, and respiratory syncytial viruses, may result in disseminated multifocal opacities. When the course of the viral infection is mild, confirmation is rarely obtained during the acute phase of the disease, but may be made by viral culture or acute and convalescent serologic studies.
Rickettsial Infection
Rocky Mountain spotted fever is a lesser known cause of pulmonary vasculitis that may lead to the appearance of multiple areas of air space consolidation and even an appearance similar to that of pulmonary edema. 333 , 365 This rickettsial infection also results in a diffuse vasculitis. It is best diagnosed by the clinical findings of a rash and central nervous system findings. A history of tick bite and an increase in antibody titers strongly support the diagnosis.
Granulomatous Infections
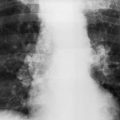
Histoplasmosis 97 ( Fig 16.4 ) is the most likely of the granulomatous infections to produce ill-defined multifocal opacities of varying sizes. This form of histoplasmosis is usually seen after a massive exposure to Histoplasma capsulatum. This organism is a soil contaminant found primarily in the Mississippi and Ohio River valleys, and histoplasmosis should be suspected when this pattern is seen in acutely ill patients from these endemic areas. A history of prolonged exposure to contaminated soil is frequently obtained and helps confirm the diagnosis. A marked rise in serologic titers is also confirmatory. The radiologic course is characterized by gradual healing of the process, involving contraction of the opacities; resolution of a large number of opacities; and the development of a pattern of scattered, more circumscribed nodules. This may precede the characteristic appearance of multiple calcifications that develops as a late stage of histoplasmosis.