Clinical presentation (attacks)
Objective clinical lesions
Additional criteria needed
2 or more
2 or more
None; clinical evidence for multiple sclerosis will suffice
2 or more
1
Dissemination in space demonstrated by MRI
Or another clinical attack implicating a different site
1
2 or more
Dissemination in time demonstrated by MRI
Or a second clinical attack
1 (monosymptomatic presentation, clinically isolated syndrome)
1
Dissemination in space demonstrated by MRI
Dissemination in time demonstrated by MRI
Or a second clinical attack
0 (primary progressive MS)
1
One year of disease progression and 2 of the following 3 criteria:
Cerebral dissemination in space
Spinal dissemination in space with 2 or more T2 lesions
Positive CSF (oligoclonal bands and/or elevated IgG index)
Source: Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997;120(Pt 11):2059–2069; Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 2009;5(5):256–266.
Dissemination in space | Dissemination in time |
Dissemination in space requires ≥1 T2 lesions in 2 or more of the following locations:
| Dissemination in time can be established in either of two ways:
|
Source: McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50(1):121–127; Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005;58(6):840–846. | |
The characteristic CSF findings in multiple include a moderate lymphocytic and monocytic pleocytosis (<20 cells/μL), autochthonous IgG production, and isoelectric focusing evidence of oligoclonal IgG bands indicating persistent antibody secretion by plasma cell clones. Approximately 95% of patients with clinically definite multiple sclerosis have positive oligoclonal bands.
Based on a growing awareness that axonal and neuronal degenerative processes are active even during the early stage of the disease, multiple sclerosis is considered a neurodegenerative disease and efforts must be made to develop biomarkers for its course that can be evaluated with multimodal techniques.
6.3.3 Treatment and Response
In recent years there have been lasting changes in therapeutic approaches to multiple sclerosis. New discoveries on pathogenesis and the development of pathogenetically based concepts have led to significant advances.
High-dose corticosteroids are a classic mainstay and are particularly useful for the treatment of optic neuritis and acute attacks.
Immunomodulatory agents such as interferon-β and glatiramer acetate have proven effective for reducing the relapse rate in RRMS (by approximately 30–35%) and also delaying disease progression and MRI lesion development in large, randomized, double-blind, placebo-controlled phase 3 trials.
Natalizumab is a humanized monoclonal antibody that binds to antigens (α4β1 integrins) on the surface of activated T-lymphocytes. It prevents the lymphocytes from migrating across the blood–brain barrier into the CNS parenchyma by binding to α4β1 integrins on the endothelial surface of arteries supplying the brain. In one placebo-controlled phase 3 trial, natalizumab showed high efficacy for the primary endpoints (68% reduction in mean annualized relapse rate, delay of disability progression). In 2006 natalizumab was approved in Europe for the treatment of patients with rapidly progressive RRMS or high disease activity unresponsive to interferon-β or glatiramer acetate.
Note
Despite its high therapeutic efficacy, natalizumab is associated with a clinically significant risk of developing progressive multifocal leukoencephalopathy ( ▶ PML), a CNS infection caused by the JC virus, which is pathogenic only in immunocompromised patients.
As of January 31, 2013, 312 cases of PML had been documented worldwide in a total of 108,300 patients exposed to natalizumab. This severe complication is most common in patients who have received natalizumab for more than 24 months, had previous immunosuppressant therapy (e.g., mitoxantrone, cyclophosphamide), or have detectable serum antibodies against the JC virus. The treatment of natalizumab-associated PML consists of discontinuing natalizumab and instituting plasmapheresis or immunoadsorption to accelerate natalizumab clearance from the circulation. ▶ IRIS is treated with corticosteroids and, if necessary, antiedema therapy and intensive medical care.
Recent phase 3 trials have shown promising efficacy for other immunomodulators, some of which have novel mechanisms of action. In the near future we may expect the approval of additional drugs and the advent of more differentiated therapies for multiple sclerosis patients.
6.4 Pathology
The hallmark of demyelinating diseases is the presence of demyelinated plaque (Prineas and McDonald 1997). Sites of predilection for plaque formation are the periventricular white matter, optic nerves, cerebellar peduncles, and spinal cord. The brainstem nuclei and cerebral cortex are less commonly affected. Myelin destruction occurs within the plaque, accompanied by a variable apoptosis of oligodendrocytes and an inflammatory reaction. The composition of the inflammatory infiltrate depends on the stage of the demyelinating process. Early, active plaque is characterized by edema and perivascular lymphocytic infiltrates composed of T-cells. As the plaque advances, the lymphocytes are replaced by lipid-laden macrophages. The chronic stage is marked by a transition to gliosis.
Earlier studies on demyelinating diseases assumed that myelin destruction was accompanied by a relative sparing of axons. However, more recent data from histopathologic studies suggest that even at a fairly early stage, demyelination is accompanied by axonal degeneration and neuronal loss. Besides focal lesions, multiple sclerosis is also associated with diffuse CNS changes that include atrophy of the white matter, brainstem, optic tract and spinal cord. Even grossly normal-appearing white matter is found on closer inspection to exhibit diffuse gliosis and perivascular inflammation.
Note
Multiple sclerosis was long regarded as a typical white-matter disease, but early involvement of the gray matter, independent of white-matter involvement, has been widely documented in histologic and imaging studies.
Two features of this gray-matter involvement are cortical atrophy and foci of significant cortical demyelination. Especially in the chronic stage, cortical demyelination appears to predominate over white-matter changes.
While the exact cause of most primary demyelinating diseases is unknown, several pathogenic mechanisms have been described for secondary demyelinating diseases:
Direct viral infection
Immune response to viral infection or vaccination
Toxic injury
Malnutrition or vitamin deficiency
6.5 Magnetic Resonance Imaging
6.5.1 Examination Technique
6.5.1.1 Conventional Magnetic Resonance Imaging
Conventional PDw, T1w and T2w turbo spin echo (TSE), and SE sequences are generally sufficient for the standard clinical MR evaluation of suspected multiple sclerosis. The standard workup should include multiplanar views and imaging after intravenous contrast administration ( ▶ Table 6.3).
Tips and Tricks
Follow-up is useful in cases of unexpected clinical deterioration, to reevaluate lesion load before treatment modification, and even in patients with a suspected second disease. Always follow the same protocol that was used for initial imaging. Routine follow-up imaging at designated intervals may employ a shortened protocol without intravenous contrast administration.
Sequence | Orientation | Slice thickness (mm) | Scan time (min) | Guidelines(1) | Comments |
SE T1w | Axial | ≤ 4 | c.2.5 | Optional | |
Three-dimensional T1w | Sagittal | 1 | c.5 | Optional | Possible alternative to axial T1w SE sequence |
FLAIR | Axial | ≤4 | c.3 | Recommended | |
Sagittal | ≤4 | c.2:10 | Recommended | Over corpus callosum | |
DWI | Axial | ≤4 | c.1:50 | – | For differential diagnosis; start simultaneous contrast administration |
Double-echo TSE image PDw/T2w | Axial | ≤3 | c.4:20 | Recommended | |
Postcontrast SE T1w | Axial | ≤4 | c.2.5 | Recommended | |
Coronal | ≤3 | c.3 | – | Over posterior fossa and corpus callosum | |
(1) Source: Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol 2006;27(2):455–461; sequences recommended for the initial diagnosis of multiple sclerosis; scan time at 1.5 T. | |||||
Owing to their high sensitivity, especially for juxtacortical white-matter lesions, axial slices are usually acquired across the whole brain using FLAIR sequences. These sequences are not suitable for the posterior cranial fossa, however, due to frequent pulsation artifacts.
T1w SE scans should be acquired at identical slice positions in RRMS patients to detect “black holes” ( ▶ Fig. 6.1), i.e., old lesions that appear as parenchymal deficits. Because black holes are also detectable after contrast administration, it is the practice at some centers to acquire a three-dimensional T1w data set with an isotropic resolution of at least 1 mm3 (called MPRAGE, 3D TFE, 3D Fast FE, 3D FGRE, or 3D SPGR by different manufacturers) instead of an unenhanced T1w SE sequence, as the three-dimensional sequence is better for evaluating concomitant atrophy.
Note
Because the corpus callosum is a site of predilection for demyelination in multiple sclerosis, sagittal T2w or FLAIR images are excellent for demonstrating the “Dawson fingers” that radiate laterally into the white matter from the upper portions of the corpus callosum ( ▶ Fig. 6.2), as well as the “fingerprints” that may appear along the inferior surface of the corpus callosum.

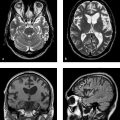
Fig. 6.1 Patient with a long history of RRMS. (a) In an unenhanced T1w sequence (3 T), the older lesion in the left periventricular white matter is approximately isointense to CSF. The signal variations within the lesion probably represent different degrees of demyelination. (b) In the corresponding FLAIR sequence, the lesions show variable hyperintensity.

Fig. 6.2 Patient with known RRMS. Sagittal T2w TSE sequence displays the radial arrangement of the periventricular hyperintensities. The lesions extending into the deep white matter are called “Dawson fingers.”
To avoid missing even the smallest lesions, especially on initial imaging, many authors recommend the use of a sagittal FLAIR sequence instead of a pure T2w sequence without an inversion pulse. Thin T2w slices of the posterior fossa (3-mm maximum slice thickness) can also contribute to the MRI detection of dissemination in space by detecting infratentorial involvement ( ▶ Fig. 6.3). Some authors recommend the use of dual-echo TSE sequences owing to their higher sensitivity in the posterior fossa; these sequences also produce PDw images as an adjunct to T2w scans.

Fig. 6.3 Acute relapse of known multiple sclerosis. Axial T2w sequence (3 T) shows a hyperintense lesion in the middle cerebellar peduncle on the right side.
Contrast-enhanced T1w sequences can detect dissemination in time on initial diagnosis, as areas of fulminant demyelination typically enhance after intravenous contrast administration. Axial scans, analogous to the FLAIR images, should be obtained, and a second plane should be imaged in thin slices to allow a detailed evaluation of the posterior fossa. Flow-compensated sequences are preferred, as they avoid pulsation artifacts from arterial and venous vessels.
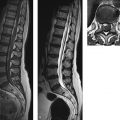
The spinal cord and optic nerves should be imaged in patients with myelitic or neuritic involvement. Spinal cord imaging should employ sagittal T2w TSE sequences and should always include T1w TSE sequences after contrast administration (3-mm slice thickness). Axial T1w and T2w sequences may also be helpful, depending on the clinical presentation and on possible lesions revealed by sagittal images. The field of view for sagittal images should not be too large, as this makes it difficult to appreciate fine details in the spinal cord. If the whole spinal column needs to be examined, it has proven helpful to divide the field of view into two or even three parts. For axial imaging, T2w SE or TSE images are preferred to T2*w gradient echo (GRE) images. While the latter are advantageous for suppressing pulsation artifacts about the cord, they also depict the internal gray- and white-matter structures of the spinal cord. Often this makes the internal structures more difficult to distinguish from demyelinated areas than on TSE images.
Tips and Tricks
Because areas of fulminant demyelination may take some time to enhance on MRI, allow at least 5 minutes between contrast injection and the acquisition of postcontrast images (e.g., by interposing the FLAIR sequence).
The PDw and T2w sequences can be acquired after contrast administration, which allows for early contrast injection during the examination. At some centers the FLAIR image is also acquired during contrast administration, although this will alter the signal characteristics of enhancing lesions.
The MRI protocol for optic neuritis also requires a departure from the standard cerebral protocol described above. The optic nerve is imaged with special thin-slice T1w and T2w SE sequences, which are tailored for maximum spatial resolution. This can be done by reducing the field of view or, if a larger field is needed, by using a higher matrix size (e.g., 512 pixels). Axial images should have a 2- to 3-mm slice thickness, and coronal images should not exceed a slice thickness of 3 mm. Axial images should be angled parallel to the optic nerve ( ▶ Fig. 6.4). Coronal slices are of greater diagnostic importance, however, as they can display the full course of the optic nerve in perpendicular sections ( ▶ Fig. 6.5). Because extensive chemical shift artifacts tend to occur at the retrobulbar level, the frequency bandwidths of the sequences should not be too low. While lower bandwidths provide a good signal-to-noise ratio, they are also much more susceptible to artifacts at fat–water tissue interfaces than sequences with a higher-frequency bandwidth. As in other protocols, postcontrast scans should generally be acquired in two planes—axial and coronal—to ensure the detection of enhancement within the optic nerve that would indicate florid inflammation. Contrast-enhanced T1w sequences with fat suppression may be helpful for detecting enhancement in axial images of the optic nerve, especially if the nerve is not fully demonstrated in the image slice.

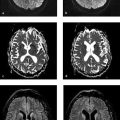
Fig. 6.4 Acute optic neuritis. Fat-suppressed, contrast-enhanced T1w TSE image angled parallel to the optic nerves demonstrates an elongated hyperintense area in the right optic nerve.

Fig. 6.5 Optic neuromyelitis. MRI shows hyperintensity and expansion of the right optic nerve, which enhances due to florid inflammation. An elongated T2 lesion is visible in the cervical spinal cord. (a) Axial T2w TSE sequence. (b) Coronal T2w TSE sequence. (c) Coronal contrast-enhanced, fat-suppressed T1w TSE sequence. (d) Sagittal T2w sequence.
6.5.1.2 New Techniques
Magnetization Transfer
The MT effect is based on an exchange process between free protons, which are used for standard MR imaging, and protons that are bound to proteins, glycoproteins, or membrane lipids and are not accessible to ordinary imaging due to their very short T2 relaxation time. These bound protons can be saturated by applying special (off-resonance) high-frequency pulses that precede the actual on-resonant high-frequency excitation pulse used for imaging. By stimulating an exchange between the two proton compartments, these prepulses can also modulate the signal from the imaging protons. As a rule, the MT effect causes a decrease in signal intensity. In intracranial imaging, this signal decrease is most pronounced in healthy white matter (35–50%), especially the corpus callosum with its densely packed myelin sheaths. Demyelination, macrophage infiltration, and axonal destruction tend to reduce the MT-related signal decrease (MT effect).
The MT effect in multiple sclerosis can be detected both in T2 lesions and in white- and gray-matter areas that are histologically affected but appear grossly normal on routine MRI. The signal change can be explained by an increased content of free protons and, in demyelination, by a reduced concentration of protons and macromolecules that are bound in the myelin sheaths. In patients with equivocal lesions, a reduction of the MT effect by more than 40% indicates that demyelination is present. Because the MT effect is increased by remyelination, it is often used to monitor treatment response in randomized controlled trials. “Background suppression” by the MT pulses, chiefly in the white matter, improves the visualization of small or weakly enhancing demyelinated areas on contrast-enhanced T1w images. This technique has not come into routine use, however, because the MT effect produces a general change in tissue contrasts and may increase pulsation artifacts. For this reason, an ordinary postcontrast image should also be obtained whenever MT imaging is used.
Diffusion-Weighted and Diffusion Tensor Imaging
DWI has become an important tool for clinical MRI in recent years. Besides the morphologic changes detected by standard MRI, DWI can assess the mobility of water protons, which are important for imaging, with a high degree of spatial resolution. Proton mobility can provide useful information on the regional microstructure of brain tissue. While proton mobility in free water is isotropic (i.e., the same in all directions), this is not the case in biologic tissues. The fiber tracts in particular tend to direct proton movements parallel to the myelinated tracts while restricting movements perpendicular to the fibers. The DTI technique is based on DWI and measures the mobility of water protons in various directions. This lets us determine quantities such as diffusion direction (tensor), degree of anisotropy (fractional anisotropy), and mean diffusivity in order to evaluate the course and integrity of the fiber tracts.
The strength of DWI is characterized by the b-value, which depends on quantities such as the amplitude of the magnetic diffusion gradient. b-values of approximately 1000 s/mm2 have proven very suitable for lesion detection. In DWI, signal intensity declines as the b-value and molecular mobility increase. The mobility of water molecules in a particular region is characterized by the apparent diffusion coefficient (ADC) value, which is determined by the strength of the signal decrease in DWI acquisitions with increasing b-values.
When the mobility of water molecules is decreased, due for example to cytotoxic edema in an acute stroke, the b-value rises and the infarcted area shows less signal decrease than its surroundings. As a result, it will appear hyperintense to its surroundings on DW images with higher b-values and hypointense in the ADC map.
An ADC map should always be generated in DWI because even with high b-values, some residual T2* weighting will still be present when echo-planar sequences are used. This effect, called “T2 shine-through,” can cause high signal on DWI that is actually due to a T2* increase rather than restricted diffusion.