CHAPTER 12 Multisystem Metastases and Assessment of Treatment Efficacy
A major focus in the management of cancer patients is the monitoring of response to treatment, whether chemotherapy, hormone therapy, or biologics. Two important issues are the accurate assessment of therapeutic efficacy and the provision of prognostic information. Clinical signs and symptoms can be subjective and difficult to evaluate because of the side effects of treatment. Serum tumor markers may lack sensitivity or specificity. Imaging offers an objective, and often quantifiable, method of measuring response. CT, MRI, positron emission tomography (PET), and bone scintigraphy have all demonstrated both advantages and disadvantages for patient monitoring and should be viewed as complementary modalities. Chemotherapeutic efficacy can be assessed in different contexts, such as neoadjuvant (i.e., presurgical) applications and in the setting of metastatic disease (measurable distant disease).
NEOADJUVANT
Neoadjuvant chemotherapy was initially applied to locally advanced breast cancer but has more recently been used in patients presenting with smaller, resectable breast cancers.1 Studies identifying the amount of breast and axillary tumor after neoadjuvant chemotherapy have demonstrated prognostic value in predicting disease-free and overall survival.2–5 In a study using fluorodeoxyglucose (FDG) PET, Bellon and coworkers found that internal mammary nodal involvement in patients with locally advanced breast cancer was predictive of regional or systemic failure.6 Modalities with exquisite anatomic and localization capabilities, such as CT and MRI, rely on a change in lesion size to assess efficacy. It is well recognized that a change in size may lag for weeks or months. CT scans are not routinely used to monitor the efficacy of chemotherapy in the neoadjuvant setting, although they may be very helpful to investigate a new finding during this time period. MRI has shown promise, but early results require larger, confirmatory studies. Dynamic contrast-enhanced MRI allows analysis of both tumor size and contrast enhancement pattern. Although these parameters may ultimately prove to be of great value in separating responders from nonresponders, underestimation of residual tumor size, comparing baseline and follow-up studies, could produce false-negative results, especially in larger tumors.7,8 In the neoadjuvant setting, imaging is used primarily to assess local response.
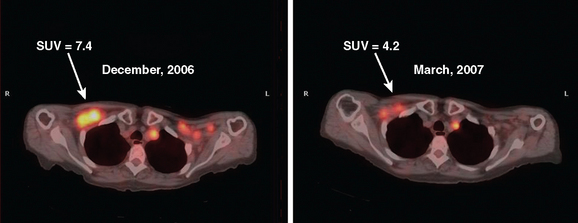
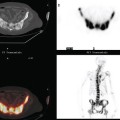
Metabolic imaging, specifically FDG PET, has shown encouraging results in the neoadjuvant setting.9–12 PET can be assessed visually or semiquantitatively. The most widely used quantitative method is measurement of the standardized uptake value (SUV) (Figure 1). This measures the amount of FDG uptake in a region of interest (i.e., tumor), divided by the injected dose corrected for patient weight. Many factors can affect this measurement, including lesion size, time interval between injection of FDG and time of imaging, and serum glucose level. Despite these and other variables, the SUV has proved useful for sequential assessment. Changes in SUV may be detected as early as after one cycle of chemotherapy. A significant fall in SUV separates responders from nonresponders and is also used prognostically. These data have been validated against histopathology.11,13
METASTATIC
Monitoring response to chemotherapy in the metastatic setting is also an evolving challenge for imaging. Supporting data from large, wellcontrolled, prospective studies are lacking for both soft tissue and bone metastases. Interest in this particular aspect of breast cancer treatment has been sparked by the continued development of new chemotherapeutic agents, as well as the significant potential toxicity of these drugs. As in the neoadjuvant setting, CT and MRI reliably provide data on lesion size changes. However, changes in size may not occur for months in patients who are responding to treatment. Additionally, various cytostatic agents may never result in a decrease in size, even if efficacious in prolonging survival. Preliminary data on the use of FDG PET are encouraging, with some authors relying on a change in SUV to separate responders from nonresponders.13–15 Significant differences have been shown between responders and nonresponders, with responders showing declining FDG uptake, as compared with minimal to no change in nonresponders. These changes have been demonstrated as early as after one cycle of chemotherapy. In the area of bone metastases, FDG PET appears to offer an advantage over conventional imaging (CI), including bone scintigraphy. Both CI and scintigraphy have difficulty assessing change in size of bone metastases; however, demonstration of significantly decreased or resolution of hypermetabolic activity on FDG PET provides valuable information.16
A possible caveat in the use of FDG PET to monitor response to therapy may be in patients treated with hormonal therapy. Initial studies have shown a transient increase in glucose use in responding patients, resulting in a “metabolic flare.” This may be due to an initial agonist effect rather than an antagonist effect of therapy. In two reports, responders showed this early increase in FDG uptake, whereas nonresponders did not.17,18
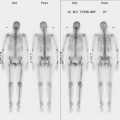
Skeletal scintigraphy is covered in Chapter 8. Although whole-body bone scanning is a sensitive test for the identification of osteoblastic metastases, inherent problems with the physiologic basis for a positive study limit its utility for assessing efficacy of chemotherapy. As discussed previously, bone-seeking radiopharmaceuticals concentrate in large part owing to localized osteoblastic activity. Even relatively small increases in osteoblastic activity result in focal hot spots, often weeks to months before these lesions become visible on plain radiography or CT. However, the healing response alone, even in the absence of viable tumor, typically elicits increased radiopharmaceutical uptake, indistinguishable from active metastases. The added issue of suboptimal specificity (i.e., inability to separate benign from malignant disease) limits the role of bone scintigraphy as a modality to assess chemotherapy response. Two exceptions would be the disappearance of previously identified bony metastases on scintiscan and the development of new sites while receiving treatment. Under these circumstances, more definitive statements can be made.
1 Bonadonna G, Valagussa P, Zucali R, et al. Primary chemotherapy in surgically resectable breast cancer. CA Cancer J Clin. 1995;45:227-243.
2 Mankoff DA, Dunnwald LK. Changes in glucose metabolism and blood flow following chemotherapy for breast cancer. Avril N, editor. PET Clinics. Philadelphia: WB Saunders; 2006;1:71-81. No. 1
3 Machiavelli MR, Romero AD, Perez JE, et al. Prognostic significance of pathological response of primary tumor and metastatic axillary lymph nodes after neoadjuvant chemotherapy for locally advanced breast carcinoma. Cancer J Sci Am. 1998;4(2):125-131.
4 Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine year results from national surgical adjuvant breast and bowel project B-18. J Natl Cancer Inst Monog. 2001;30:96-102.
5 McCready DR, Hortobagyi GN, Kau SW, et al. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg. 1989;124:21-25.
6 Bellon JR, Livingston RB, Eubank WB, et al. Evaluation of the internal mammary lymph nodes by FDG-PET in locally advanced breast cancer (LABC). Am J Clin Oncol. 2004;27(4):407-410.
7 Rieber A, Brambs HJ, Gabelmann A, et al. Breast MRI for monitoring response of primary breast cancer to neoadjuvant chemotherapy. Eur Radiol. 2002;12(7):1711-1719.
8 Martincich L, Montemurro F, De Rosa G, et al. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat. 2004;83(1):67-76.
9 Wahl RL, Zasadny K, Helvie MA, et al. Metastatic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol. 1993;11:2101-2111.
10 Bassa P, Kim EE, Inove T, et al. Evaluation of preoperative chemotherapy using PET with {fluorine-18} fluorodeoxyglucose in breast cancer. J Nucl Med. 1996;37:931-938.
11 Schelling M, Avril N, Nahrig J, et al. Positron emission tomography using [18F]–fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689-1695.
12 Biersack HJ, Palmedo H. Locally advanced breast cancer: is PET useful for monitoring primary chemotherapy? J Nucl Med. 2003;44(11):1815-1817.
13 Jansson T, Westlin J, Ahlstrom H, et al. Positron emission tomography studies in patients with locally advanced and/or metastatic breast cancer: a method for early therapy evaluation? J Clin Oncol. 1995;13:1470-1477.
14 Gennari A, Donati S, Salvador B, et al. Role of 2-[18F]–fluorodeoxyglucose (FDG) positron emission tomography (PET) in the early assessment of response to chemotherapy in metastatic breast cancer patients. Clin Breast Cancer. 2002;1:156-161. discussion 162–163
15 Dose Schwartz J, Bader M, Jenicke L, et al. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG-PET imaging. J Nucl Med. 2005;46(7):1144-1150.
16 Hoh CK, Schiepers C. 18-FDG imaging in breast cancer. Semin Nucl Med. 1999;29:49-56.
17 Dehdashti F, Flanagan FL, Mortimer JE, et al. Positron emission tomographic assessment of “metabolic flare” to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med. 1999;26:51-56.
18 Mortimer JE, Dehdashti F, Siegel BA, et al. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797-2803.