Musculoskeletal, Soft Tissue, and Miscellaneous Applications
Beyond the well-known primary applications of emergency ultrasound lies a veritable smorgasbord of clinically useful ultrasound applications. Some of these applications allow emergency care providers to rapidly evaluate and better manage common clinical problems. This chapter will focus on eight applications of point-of-care ultrasound that are useful in the acute care setting. These include (1) evaluation of abdominal wall pain and masses; (2) airway assessment; (3) evaluation of bony cortices for rapid fracture diagnosis and postreduction alignment; (4) subcutaneous foreign body diagnosis and localization; (5) imaging of selected tendons, joints, and muscles for common musculoskeletal complaints; (6) diagnosis of salivary gland disease; (7) point-of-care detection of maxillary sinusitis; and (8) evaluation of soft tissue infections, particularly for detection and accurate localization of subcutaneous abscesses prior to drainage.
 ABDOMINAL WALL
ABDOMINAL WALL
CLINICAL CONSIDERATIONS AND INDICATIONS
A surprisingly wide range of pathologic processes can occur in the abdominal wall, and a patient’s abdominal pain may, on occasion, be discovered due to a lesion or defect within this anatomic region. Since the area of anatomic interest is quite superficial and free of shadowing artifacts, it is well suited to sonographic evaluation with a linear array transducer. When a palpable or indistinct abdominal wall mass is found on physical examination, or when a focal area of abdominal wall tenderness is encountered, a point-of-care ultrasound examination of the affected area may help provide immediate answers to a number of clinical questions. Is the region of tenderness due to a lesion within the abdominal wall itself or does it appear that an underlying structure (e.g., a metastatic lesion in the liver) is causing the discomfort? If a lesion is present, where is it and what are its sonographic characteristics? Is it solid, cystic, hypo, or hyperechoic, and is it a vascular structure? Is a fluid collection present, and if so, is the fluid simple or complex? Is a fascial defect noted in the abdominal wall, and if present, is a loop of bowel seen passing through the defect? Armed with the additional anatomic knowledge of the site and character of the sonographic findings, as well as the clinical history, the provider can then pursue a more targeted workup.
Ultrasound examination of the abdominal wall can provide valuable information when the diagnosis of an abdominal wall hernia is unclear. In one clinical series, 39% of 144 patients with an abdominal wall mass of unclear etiology (with or without pain) were found to have a hernia.1 Incisional hernias occur as a delayed complication in up to 4% of abdominal surgeries2, and ultrasound can sometimes detect the fascial defect early in its development. While many abdominal wall hernias are apparent on clinical examination alone and do not require sonographic evaluation for diagnosis, others can be difficult to diagnose because the fascial defect is small and difficult to appreciate clinically. The fascial defect in a Spigelian hernia (also known as an interstitial hernia) will be found along the lateral border of the rectus muscle and a focal defect will be present in the aponeuroses of the transversus abdominis and internal oblique muscles, but not in the aponeurosis of the external oblique muscle. Since the fascial defect lies beneath the external oblique aponeurosis, the defect may not be apparent on clinical examination. A Spigelian hernia will typically be found where the lateral rectus sheath intersects with the inferior margin of the rectus sheath at a region termed the arcuate line, located about halfway between the umbilicus and the pubis. Signs and symptoms of a Spigelian hernia can be nonspecific and the pain may be poorly localized. Peak incidence is at age 50, with men and women affected equally.3 Additionally, ultrasound can play an important role in the evaluation of patients with inordinate pain or excessive swelling of the abdominal wall in the postoperative period after a herniorrhaphy.1
The femoral region may be host to a wide range of pathologic and postoperative entities ranging from inguinal and femoral hernias, reactive and metastatic lymph nodes, lipomas, abscesses, hematomas, seromas, lymphomas, soft tissue sarcomas, vascular bypass grafts, and pseudoaneurysms.4 Ultrasound examination of the groin can narrow the differential diagnosis and help differentiate among the many pathologic processes that occur in this anatomic region. Ultrasound is more accurate than the physical exam for distinguishing inguinal adenopathy from other inguinal pathology.2
A patient’s abdominal pain is sometimes discovered to be due to a spontaneous or posttraumatic rectus sheath hematoma, most frequently caused by sudden vigorous abdominal contractions in the setting of a seizure, a coughing or sneezing paroxysm, direct trauma, or recent surgery. Older patients on anticoagulant therapy are most prone to this malady. In one series of 16 cases, 73% were on anticoagulant therapy and the mean age was 64.5 years old.5 Bleeding may occur because of rupture of an epigastric artery or vein or because of a tear of the rectus muscle fibers.2 The resulting hematoma remains confined to the rectus sheath.
Abdominal wall endometriosis can occur at the site of a previous C-section or laparotomy and should be considered in the differential diagnosis of women presenting with recurrent focal abdominal wall pain near a surgical scar during menses; the frequency of this disorder is estimated at 0.8% of all C-sections.6 In one series of 28 patients scar endometrioma sizes ranged from 0.7 to 6 cm and the average time since the last C-section ranged from 40 to 66 months. In the 12 patients with large scar endometriomas (3–6 cm), the hypoechoic lesions exhibited increased vascularity, solid and cystic portions, occasional fistulous tracts, and irregular shapes when compared with smaller scar endometriomas.7 Although the sonographic finding of a hypoechoic mass within the region of the operative scar is nonspecific, this finding coupled with a characteristic history can help make the diagnosis.8
Other abdominal wall masses such as lipomas, sebaceous cysts, subcutaneous abscesses, cutaneous metastases, a primary malignant melanoma, hemangiomas, and pseudoaneurysms of the epigastric artery may all occur in the abdominal wall and should also be included in the differential diagnosis of a palpable or tender abdominal wall mass. Ultrasound has been used to help localize the injection port of intrathecal drug delivery pumps whose location in the abdominal wall cannot be found by physical examination.9
Ultrasound also improves the performance characteristics of selected nerve blocks of the abdominal wall (the transversus abdominis plane or TAP block, as well as ilioinguinal nerve blocks), and has been used to guide injection therapy of entrapped abdominal wall cutaneous nerves at the lateral border of the rectus abdominis.10–12
ANATOMICAL CONSIDERATIONS
The abdominal wall is composed of skin, subcutaneous tissue of varying thickness depending on patient habitus, muscular layers that also vary in thickness with patient habitus and conditioning, and finally a layer of extraperitoneal fat. The muscular layers are enclosed in fibrous fascial sheaths. The fascial sheaths or aponeuroses of the three lateral abdominal wall muscles (the external oblique, the internal oblique, and the transverses abdominis muscles) combine to form a thickened fascial layer known as the Spigelian fascia in the paramedian region just lateral to the paired midline rectus muscles. The region along the lateral border of the rectus muscles extending from the costal margin to the pubic bone is referred to as the linea semilunaris or Spigelius line. Moving medially, the Spigelian fascia divides into two layers to form the anterior and posterior rectus sheaths that surround the rectus muscles. In the midline, the anterior and posterior rectus sheaths from each rectus muscle combine and fuse into a single central fascial layer known as the linea alba. In long axis, the rectus muscles appear as paired bundles of muscle tissue with the muscle fibers aligned in a sagittal orientation, interrupted by three transversely oriented tendinous intersections. In cross section, the rectus muscles are ovoid in profile. Of note, the posterior layer of the each rectus sheath ends approximately midway between the umbilicus and the pubic symphysis. The thickened inferior edge of the rectus sheath at this level forms an anatomic region termed the arcuate line. Below the arcuate line, the posterior layer of the rectus sheath is composed only of a thin layer of tissue known at the transversalis fascia.
The anatomy of the inguinal region is more complex and the region immediately adjacent to the inguinal ligament is the area of anatomic interest. The inguinal ligament represents the thickened inferior border of the aponeurosis of the external oblique muscle and is located between the anterior superior iliac spine and the pubic tubercle of the pelvis. Beneath the inguinal ligament lie three important bony prominences. Moving medially from the laterally situated anterior superior iliac spine, the next bony ridge encountered will be the anterior inferior iliac spine. Continuing further medially, the large curved bony ridge of the iliopubic eminence will be noted. This ridge corresponds to the anterior rim of the acetabulum. Medial to the iliopubic eminence lies the bony prominence of the pubic crest, about 1 cm medial to the pubic tubercle. The iliopsoas muscle runs beneath the inguinal ligament in the space between the anterior superior and inferior iliac spines and the iliopubic eminence. Medial to lateral, the common femoral vein, the common femoral artery, and the femoral nerve are found just anterior to the iliopubic eminence. Lymph nodes can be found on either side of this neurovascular bundle. The deep inguinal ring lies superficial to the inguinal ligament in the region above the femoral vessels. From there, the inguinal canal courses medially and inferiorly toward the superficial inguinal ring that is found in close proximity to the pubic crest, still superficial to the inguinal ligament.
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
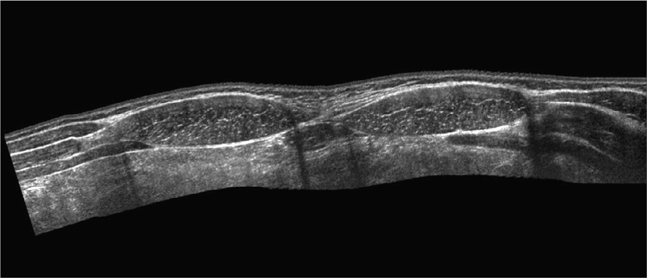
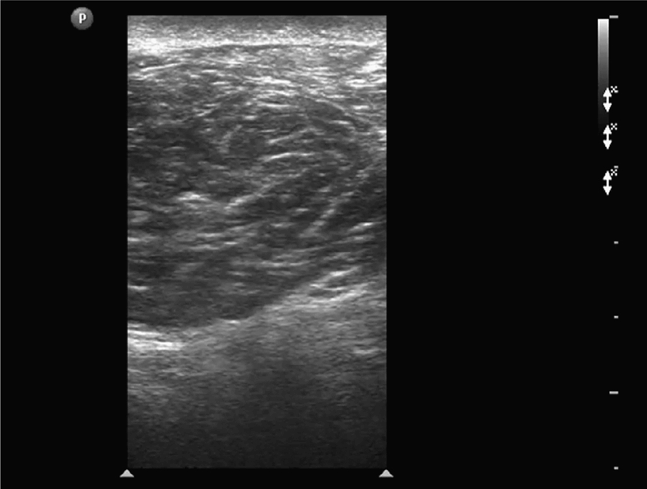
The abdominal wall is divided into three sonographically distinct regions.4 Since the anatomic structures of interest are all superficially located, a high-frequency linear array transducer is best suited to this type of examination. If extended-field-of-view or panoramic imaging software is available, this feature can be used to demonstrate the lesion’s anatomic relationship with adjacent structures in the abdominal wall (Figure 18-1).
Figure 18-1. Extended-field-of-view or panoramic view of the upper anterior abdominal wall musculature. The external oblique, internal oblique, and transversus abdominis muscles can be seen just lateral to the rectus muscles in the upper abdomen.
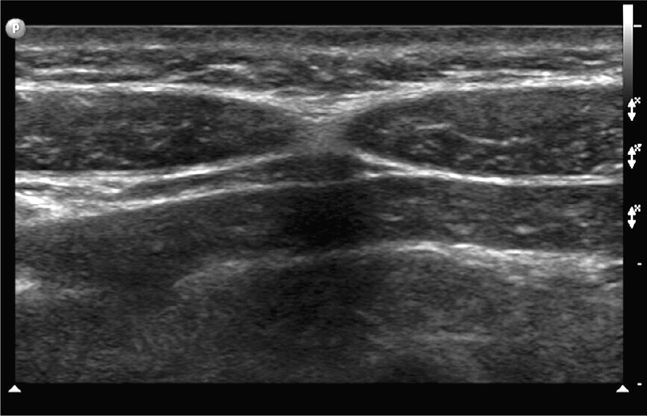
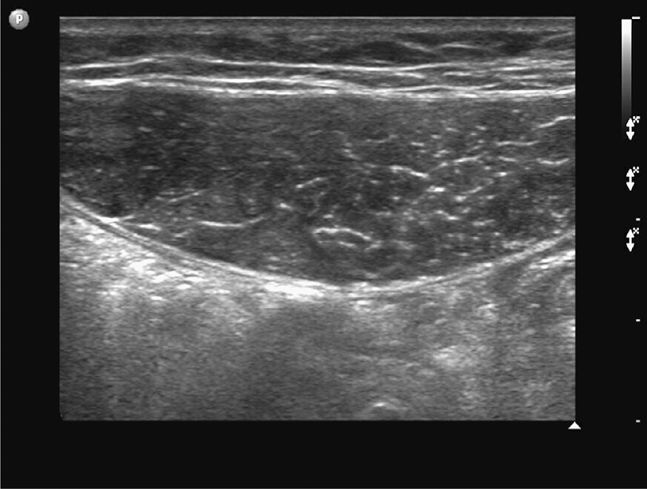
The midline region is best scanned in short axis. The linea alba (representing the midline confluence of the anterior and posterior fascial sheaths from each rectus muscle) appears beneath the skin and subcutaneous tissue of the midline abdomen as a horizontally oriented and somewhat hyperechoic and thickened line. The linea alba is surrounded on either side by the hypoechoic triangular medial portions of each rectus muscle (Figure 18-2). The rectus muscles appear hypoechoic and speckled in short axis (Figure 18-3) and hypoechoic and striated in long axis. The anterior and posterior rectus sheaths appear as thin hyperechoic lines surrounding the muscle bundles. The underlying peritoneal interface will usually be apparent on real-time scanning. The adjacent anterior bowel wall surface appears hyperechoic, and gliding of the bowel is usually noted with respiration or with bowel peristalsis. Comet-tail artifacts, or dirty shadowing, arising from pockets of admixed air and fluid in the bowel loops may also be seen (Figures 18-1 and 18-3).
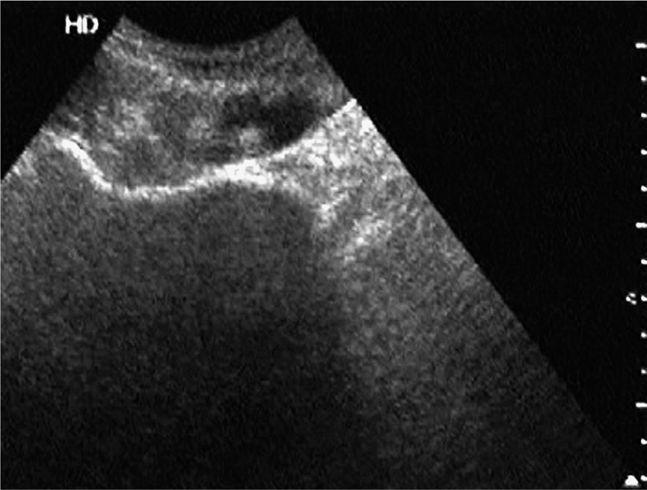
Figure 18-2. Transverse sonogram of the linea alba and adjacent rectus muscles. Skin and subcutaneous tissue are seen in the near field. The linea alba appears as a thickened, somewhat echogenic horizontal region in the midline. The hypoechoic conical or triangular regions on either side of the linea alba represent the medial portions of the adjacent rectus muscles.
Figure 18-3. Transverse sonogram of a normal left rectus muscle above the umbilicus. The rectus muscle is seen as an ovoid, hypoechoic, and somewhat speckled structure in short axis, outlined by the echogenic anterior and posterior layers of the rectus sheath. In long axis, the muscle tissue appears striated.
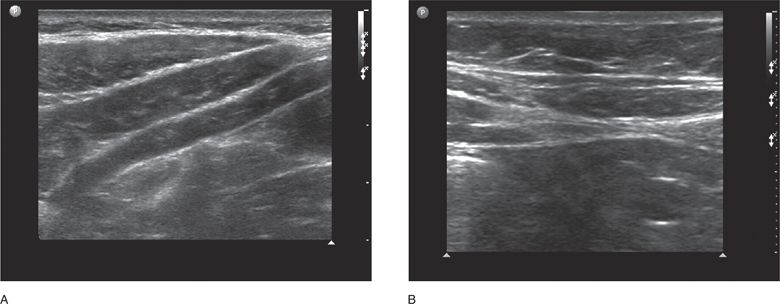
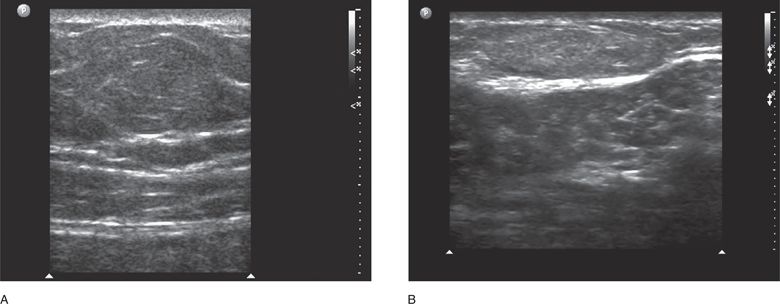
In the paramedian region, the sonographic area of interest is at the lateral border of the rectus muscle at the confluence of the aponeuroses of the lateral abdominal wall muscles. In the paramedian region, the fascial layers surrounding the three lateral wall muscles (external oblique, internal oblique, and transverse abdominal) are conjoined into one fascial layer called the Spigelian fascia. Therefore, the landmarks for ultrasound of the paramedian region are the lateral border of the rectus muscle, the Spigelian facia, and the medial border of the three lateral wall muscles (Figure 18-4A and B). Spigelian hernias occur in the Spigelian fascia between the umbilicus and the pubis.
Figure 18-4. Oblique sonogram of the right paramedian upper abdominal wall just lateral to the rectus muscle (A). The external oblique, internal oblique, and transversus abdominis muscles appear as a succession of three hypoechoic layers surrounded by their respective hyperechoic fascial sheaths or aponeuroses. As they approach the rectus muscle, they taper to form the Spigelian fascia. More inferiorly (B) the Spigelian fascia is seen as a thickened hyperechoic line representing the combined aponeuroses of the three lateral abdominal wall muscles as they approach and then split to form the anterior and posterior rectus sheaths. The lateral portion of the right rectus muscle can be seen on the right side of the image.
In the inguinal region, the area of sonographic focus will be along an oblique plane between the palpable bony landmarks of the anterior superior iliac spine and the pubic crest. The region should be scanned in a series of successive parallel planes several centimeters above and below the inguinal ligament. In the normal patient, the hypoechoic iliopsoas muscles will be seen occupying the region bounded by the anterior superior iliac spine laterally, the anterior inferior iliac spine below, and the edge of the iliopubic eminence medially. The hyperechoic curve of the iliopubic eminence will be noted just beneath the anechoic femoral vessels (Figure 18-5).
Figure 18-5. Oblique sonogram of the inguinal region with a curved array transducer. The scan plane is along the inguinal ligament. The curved hyperechoic line represents the shape of the bony pelvis beneath the inguinal ligament. The anterior superior iliac spine is not seen in this image and lies just beneath the skin off to the left of the sonogram. The first bony convexity seen on the left side of the image represents the anterior inferior iliac spine. The next convexity is somewhat shallower and more elongated, is seen on the right side of the sonogram, and represents the iliopubic eminence (corresponding to the anterior rim of the acetabulum). Posterior acoustic shadowing is seen beneath these bony ridges. The hypoechoic femoral vessels are seen in short axis just above the curve of the iliopubic eminence. The iliopsoas muscle occupies the region to the left of the femoral vessels. The upsloping bony ridge that leads to the pubic tubercle is seen beneath the femoral vessels.
COMMON AND EMERGENT ABNORMALITIES
Postoperative Abdominal Wound Evaluation
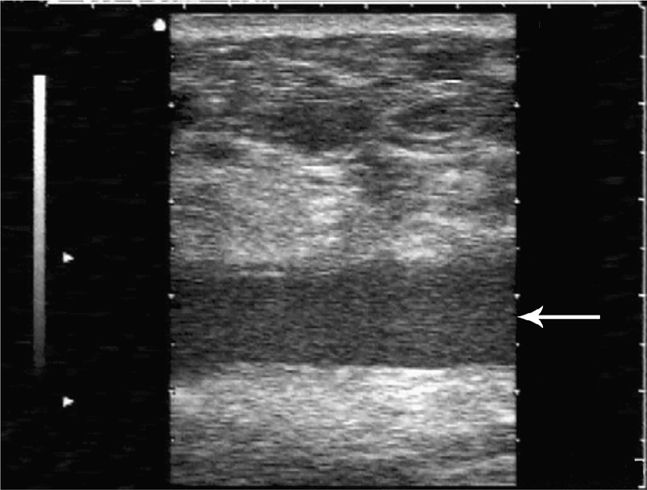
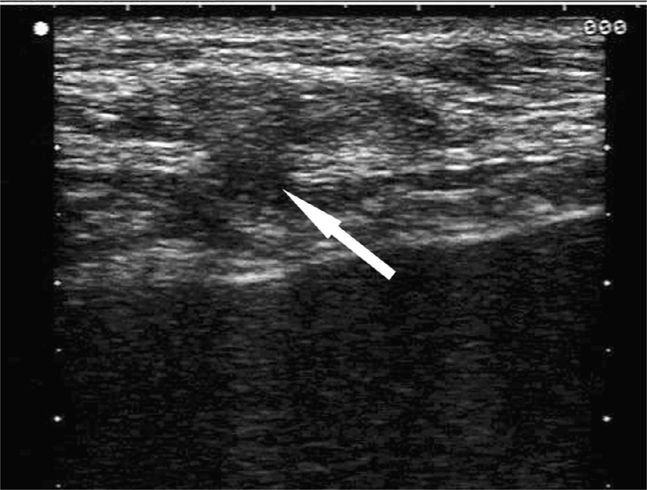
As noted earlier, a wide range of postoperative pathology may occur in the abdominal wall. A wound abscess will typically appear as a hypoechoic, sometimes isoechoic, fluid collection at or near the surgical site with clinical signs suggesting that a wound infection is present. Swirling of the abscess fluid may be noted with gentle transducer pressure on the site. Deeper abscesses may be connected by a thin column of fluid rising up to the skin surface. A postoperative seroma will manifest as an anechoic collection of easily compressible fluid with no associated clinical signs to suggest infection (Figure 18-6). Liquefying hematomas will often exhibit both simple and complex features with layering.
Figure 18-6. Transverse sonogram of an abdominal wall seroma in a patient several months after a hernia repair. A hypoechoic fluid collection (arrow) is seen beneath the subcutaneous tissues and is easily compressible. No clinical signs of infection were present.
Lymph Node
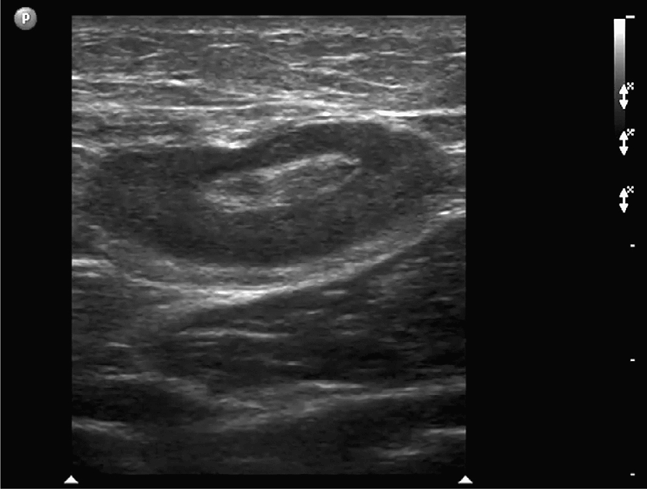
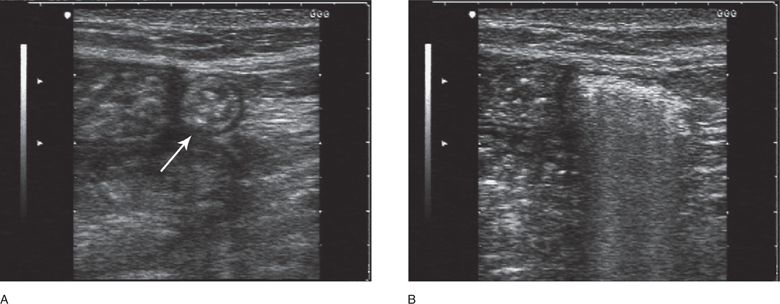
Focal tenderness in the inguinal region may be due to a reactive inguinal lymph node. A lymph node will appear as a lobulated elliptical structure in long axis, hypoechoic at the periphery with a variably hyperechoic fatty central hilum (Figure 18-7).
Figure 18-7. Long-axis sonogram of an inguinal lymph node. The oval-shaped lymph node appears hypoechoic at its periphery and echogenic at its fatty hilum. This patient’s groin tenderness was attributable to a reactive adenopathy and not a hernia.
Rectus Sheath Hematoma
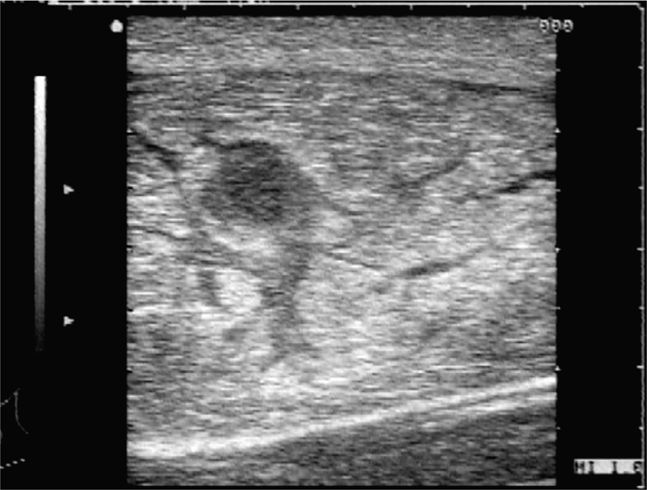
On occasion, a patient may present with a focal region of abdominal tenderness and swelling due to a rectus sheath hematoma. Sonographically, the normally homogeneously hypoechoic rectus muscle will appear diffusely hyperechoic from hemorrhage into the muscle, and a focal homogeneous fluid collection consistent with a hematoma may also be present (Figure 18-8).
Figure 18-8. Transverse sonogram of a rectus sheath hematoma. The normally hypoechoic rectus muscle appears hyperechoic and quite thick in this patient. In the center, there is a hypoechoic region consistent with an inferior epigastric artery aneurysm. The hemorrhage dissects through the muscle tissue but is contained within the rectus sheath.
Hernia
A Spigelian hernia will appear as a hypoechoic fascial defect at or near the junction of the linea semilunaris and the arcuate line. A bowel loop may be seen extending laterally under the external oblique muscle. A small epigastric hernia will appear as a hypoechoic fascial defect in the linea alba; visualization of peristaltic movements in the herniated bowel loop during real-time scanning will help confirm that a hernia is indeed present (Figure 18-9). Seen in cross section, a herniated loop of small bowel will have a rounded target-like appearance with a hypoechoic outer muscular layer, followed by a hyperechoic mucosal layer and, on occasion, strongly reflective central echoes that arise from admixtures of air and fluid in the bowel lumen (Figure 18-10). In long axis, a linear region of “dirty shadowing” and reverberation artifacts may be seen (Figure 18-10B). When obstructed, small bowel loops will appear as dilated hypoechoic tubular fluid-filled structures with prominent hyperechoic valvulae conniventes.
Figure 18-9. Transverse sonogram of an epigastric hernia. The patient had localized tenderness of the midline abdominal wall in the epigastric region but no fascial defect was appreciated clinically. A hypoechoic fascial defect (arrow) is seen on the sonogram in the otherwise echogenic linea alba. The hypoechoic mushroom-shaped region around and above the lesion represents a loop of small bowel that has herniated through the defect. On real-time imaging, peristalsis of the bowel loop was appreciated.
Figure 18-10. Transverse (A) and sagittal (B) sonograms of a small ventral hernia. A herniated loop of small bowel is seen within the abdominal wall between a fascial defect in the linea alba (to the right of the image) and the medial border of the rectus muscle (to the left). In short axis, the bowel segment has a characteristic circular and target-like appearance (arrow). In long axis, the sonographic pattern is one of “dirty shadowing” and reverberation artifacts.
Lipomas and Sebaceous Cysts
These masses typically appear as rounded or ovoid structures in the subcutaneous tissues. Palpation of the lesion will guide the clinician to the region of sonographic interest. Lipomas appear similar in echotexture to the surrounding subcutaneous tissue. A subtle curved region of echogenicity will outline the border of the lipoma in what otherwise appears to be a homogenous layer of subcutaneous tissue (Figure 18-11) Angiolipomas exhibit somewhat increased echogenicity. A sebaceous (or epidermoid) cyst appears as a heterogeneous hypoechoic cystic mass, filled with a fatty cheese-like material (Figure 18-12). If the contents extravasate into the adjacent soft tissues, an abscess will frequently form.
Figure 18-11. Transverse sonogram of abdominal wall lipomas. A firm and somewhat tender mass was appreciated clinically in both cases, but its etiology was unclear. The subtle curved outline of an ovoid structure is seen in the near field in both images. (A) The echogenicity of the lesions is similar to that of the fatty tissue in which it resides, consistent with the sonographic appearance of a lipoma. (B) The hyperechoic fascial layer below is distorted by the presence of the lipoma above.
Figure 18-12. This abdominal wall mass was noted to be somewhat rubbery, somewhat deformable with pressure, with minimal tenderness to palpation. As opposed to the homogeneous appearance of a lipoma, this lesion appears as a discrete but sonographically heterogeneous ovoid mass. The sonographic characteristics are typical of a sebaceous cyst.
COMMON VARIANTS AND SELECTED ABNORMALITIES
Endometrioma
An abdominal wall endometrioma will be found at the site of a prior caesarean section or laparotomy and will appear as a solid hypoechoic mass and scattered internal echoes similar to the endometriomas that occur in the abdominal cavity.8 An undescended testicle will appear as a homogenous mass smaller in size but similar in echotexture to a normal testicle, with its long axis parallel to the inguinal canal.
Pseudoaneurysm
A pseudoaneurysm represents an area of fibrous encapsulation around a pulsatile and expanding hematoma that occurs from arterial bleeding into adjacent soft tissue. Because there is a persistent communication between the vessel and the fluid space, to-and-fro flow will be noted between the mass and the adjacent artery and characteristic echogenic swirls will be seen on color Doppler examination. In contradistinction to a true aneurysm, the neck of a pseudoaneurysm is narrow.
PITFALL
The major sonographic pitfalls of abdominal wall imaging are failure to consider a malignant etiology for any homogeneously hypoechoic solid lesion, especially in the groin, and failure to consider a vascular etiology for an anechoic lesion, particularly if aspiration is considered.
 AIRWAY
AIRWAY
CLINICAL CONSIDERATIONS
The anatomic structures of the larynx and upper airway are superficially located and well suited for point-of-care sonographic assessment. Although airway ultrasound techniques are not widely used, there is growing evidence that ultrasound can provide valuable information about the anatomy of the upper airway and can be used to confirm proper placement of an endotracheal tube.
CLINICAL INDICATIONS
The indications for using point-of-care ultrasound for airway management are
1. Preintubation assessment of the upper airway
2. Assessment of anatomic structures for surgical airway management
3. Postintubation confirmation of endotracheal tube placement
4. Assessment of vocal cord function
5. Evaluation for epiglottitis
Preintubation Assessment of the Upper Airway
Airway ultrasound has been shown to be a useful tool for preintubation assessment in both adult and pediatric patients. Sonographic measurements of anterior soft tissue thickness at the level of the hyoid bone and thyrohyoid membrane can help predict difficult direct laryngoscopy better than clinical screening tests. Sonographic measurements of infrahyoid airway structures have been found to correlate well with CT or MRI.13 Ultrasound imaging of the width of the air column at the level of the cricoid cartilage was found to have a correlation coefficient of 0.99 with MRI measurements taken in the same group of 19 patients. This parameter could help clinicians estimate proper endotracheal tube size and avoid the complications that occur when an excessively large endotracheal tube is employed. In a study of 192 pediatric patients 1 month to 6 years of age, sonographically measured subglottic airway diameter was better predictor of proper endotracheal tube size than standard age and height based formulas.14
Assessment of Anatomic Structures for Surgical Airway Management
Ultrasound of the upper airway can also play an important role when placement of a percutaneous cricothyrotomy or tracheostomy is contemplated. Surface landmarks are notoriously unreliable, especially in obese patients. In one review, percutaneous identification of the cricothyroid membrane by 18 anesthesiologists was found to be poor. Only 30% of 108 landmark assessments were located over the cricothyroid membrane and only 10% were over the desired target site.15 In a study of percutaneous tracheotomies performed by 50 anesthesiologists on an inanimate model with unidentifiable anterior neck anatomy, ultrasound led to a significant increase in procedural success as well as a significant decrease in time to successful cannula placement (57 seconds vs. 110 seconds).16 Also, a large human study showed that the mean time for emergency physicians to accurately visualize the cricothyroid membrane with ultrasound was 24 +/– 20 seconds.17
Postintubation Confirmation of Endotracheal Tube Placement
Numerous studies discuss the role of ultrasound for endotracheal tube placement confirmation. Recognition of the characteristic sonographic patterns of endotracheal or esophageal intubation on a transverse or longitudinal view of the trachea can be of significant clinical value for rapid identification of tracheal versus esophageal intubation.18 Real-time imaging of the trachea on a transverse view at the level of the cricothyroid membrane was reported to be 99.7% sensitive and 97% specific for detection of endotracheal intubation. Real-time imaging during the intubation was found to be superior to a static imaging technique performed after the intubation. Evaluation of the static imaging technique alone revealed notably improved test characteristics when images were obtained at a suprasternal location (97% sensitivity and specificity) rather than at the cricothyroid membrane (73% sensitivity and 56% specificity).19 In another study, transverse imaging just superior to the suprasternal notch confirmed tracheal versus esophageal tube placement in 150 patients with 100% sensitivity and specificity within 3 seconds of tube insertion.20 In a cadaver model, a longitudinal scan plane was employed at the level of the cricothyroid membrane. Dynamic imaging was found to be 97% sensitive and 100% specific for endotracheal tube position confirmation. In contrast, static imaging at that site after the endotracheal tube had been placed was noted to be only 51% sensitive for confirming endotracheal tube location, which is consistent with other studies. In a randomized controlled trial, a transverse scan plane just above the suprasternal notch was employed dynamically during intubation, and correctly identified tracheal or esophageal intubations in all cases.21 In pediatric patients, visualization of widening of the glottis with endotracheal tube passage and identification of lung sliding with initial ventilation were found to be reliable indicators of tracheal tube placement.22 In one study, the combination of transverse dynamic ultrasound imaging at the cricothyroid membrane and lung sliding with initial ventilation was found to be 100% sensitive and 100% specific for endotracheal tube placement.23
Because of the large acoustic impedance mismatch between soft tissue and the air-filled trachea, visualization of an endotracheal tube within the airway may be difficult unless it is in direct contact with the tracheal wall. When a saline or foam-filled endotracheal tube cuff is utilized, the cuff will be in contact with the trachea and will exhibit a distinct sonographic pattern that assists in its identification. This technique was investigated in a series of 24 intubated patients and reached the following conclusions: (1) the saline or foam-filled cuff was best visualized in a long-axis view, (2) a slight longitudinal to-and-fro motion of the endotracheal tube further enhanced visualization of the cuff, and (3), when the cuff was visualized at the level of the suprasternal notch, the endotracheal tube was ideally situated midway between the vocal cords and the carina. The study concluded that this sonographic technique could be clinically useful for rapid assessment of endotracheal tube position in any situation where endotracheal tube movement, near extubation, or endobronchial intubation might have occurred.24
Ultrasound may also be used for secondary confirmation of endotracheal tube position either by direct observation of diaphragm motion or by identification of lung sliding during ventilation. One study of 59 emergently intubated patients ranging from newborn to 17 years of age utilized real-time B- and M-mode ultrasound and a subxiphoid window to evaluate diaphragm motion during ventilation. Of the 59 patients, 49 tracheal intubations, 2 esophageal intubations, and 8 right mainstem intubations were correctly identified with ultrasound. The authors concluded that ultrasound imaging of diaphragm motion was a “useful, quick, noninvasive, portable, and direct anatomic method for assessment of endotracheal tube position.”25 Diaphragmatic ultrasound has not been found to be a reliable indicator to distinguish mainstem endotracheal intubation, with only 50% specificity in one review.26
Using a cadaver model and a 4–2 MHz microconvex transducer, the identification of the lung sliding sign as a predictor of endotracheal tube placement was evaluated with 68 intubations in 9 cadavers.27 For differentiating esophageal versus tracheal intubation, the sensitivity was 95–100% and the specificity was 100%. Visualization of lung sliding to rule out or identify mainstem intubation has not been well studied, but is used extensively in clinical practice. However, the unilateral absence of lung sliding in patients with mainstem intubation should not be confused with a pneumothorax.28
Assessment of Vocal Cord Function
A real-time image of the vestibular folds (the false vocal cords), the vocal folds (the true vocal cords), and the arytenoids can be obtained by scanning transversely though the thyroid cartilage. Ultrasound has been found to be a useful tool for evaluation of vocal cord function by a number of investigators.29–31
Evaluation for Epiglottitis
Ultrasound has also been used to assess the anteroposterior (AP) thickness of the epiglottis. One study examined 100 normal subjects using a subhyoid window and a transverse scan plane, and the epiglottis was visualized in all cases. There was little variation in the AP diameter of the normal adult epiglottis, with an average AP dimension of 2.39 ±0.15 mm in this report.32 A long-axis view of the epiglottis may be obtained from either the midline or a paratracheal location at the level of the thyrohyoid membrane, but successful visualization of the epiglottis with this technique was noted to be only 71% compared with 100% when a transverse transducer orientation was employed. Another study touted the use of a long-axis sonographic technique as being a safe and practical way to noninvasively assess a patient for epiglottitis at the bedside. The study described a sonographic finding that they called the “alphabet P sign,” the ultrasound equivalent to the “thumb print sign” seen on the lateral neck radiograph.33 The use of ultrasound for rapid point-of-care assessment of a patient with suspected epiglottitis appears to be a promising technique.
ANATOMICAL CONSIDERATIONS
The thyroid and cricoid cartilages, the cricothyroid membrane, and the upper trachea are located within the superficial subcutaneous tissues of the anterior midline of the neck. The thyroid cartilage is composed of two broad rectangular laminae that meet in the anterior midline at about a 90° angle. Superiorly, the thyroid cartilage attaches to the hyoid bone via the thyrohyoid membrane. Posteriorly, the superior and inferior horns of the thyroid cartilage connect the thyroid cartilage with the hyoid bone and cricoid cartilages, respectively. Inferiorly and anteriorly, the thyroid cartilage connects to the cricoid ring via the cricothyroid ligament or membrane; in an adult, it averages about 2 × 1 cm in size. A V-shaped gap separates the upper aspects of the thyroid laminae in the midline and the base of this gap forms the superior thyroid notch or the laryngeal prominence. The strap muscles (the sternohyoid, omohyoid, and thyrohyoid muscles) lie just anterior to the thyroid cartilage. The cricothyroid muscles extend from the lower border of the thyroid cartilage to the lower aspect of the cricoid ring and surround the anterolateral portions of the cricothyroid membrane and cricoid cartilage. The thyroid gland surrounds the lateral portions of the cricoid ring, and extends superiorly to the lower border of the thyroid cartilage and anteriorly over the upper tracheal cartilages. The narrow rectangular midline segment of the thyroid gland is known as the thyroid isthmus.
The vestibular folds (also known as the false vocal cords or ventricular folds) are composed of a thick fold of mucous membrane and connective tissue. They lie just above and protect the more delicate vocal cords below. The vocal folds (or true vocal cords) are composed of the vocal ligaments medially and the laterally adjacent vocalis and thyroarytenoid muscles. They are covered by a mucous membrane and extend from the level of the mid-thyroid cartilage anteriorly to the paired arytenoid cartilages posteriorly. The arytenoids rest on the broad posterior cricoid ring and are attached to the thyroarytenoid muscles that adduct the vocal folds. The midline gap between the vocal ligaments is referred to as the rima glottidis.
The base of the epiglottis attaches to the upper border of the thyroid cartilage via the thyroepiglottic ligament; more superiorly the hyoepiglottic ligament provides the anterior support for the epiglottis. A preepiglottic fat pad separates the epiglottis from the thyrohyoid membrane. The epiglottis approaches its widest dimension just below the level of the hyoid bone.
The cricoid cartilage is the only complete ring of cartilage around the trachea and attaches distally to the first tracheal ring via the cricotracheal ligament. The upper five or six tracheal rings of the trachea lie just beneath the skin in the region between the cricoid cartilage and the lower aspect of the suprasternal notch. The diameter of the airway at the level of the cricoid ring governs the choice of endotracheal tube size, as this is the narrowest point in the upper airway.
TECHNIQUE AND NORMAL ULTRASOUND FINDINGS
Transducers
The superficial structures in the upper airway are best imaged with a high-frequency linear array, or hockey stick transducer. Use the short-axis midline, long-axis midline, or long-axis paratracheal views, depending on the airway application being performed. The length of the transducer face may limit its utilization in long axis if the neck is short or the transducer face is long. Generous application of ultrasound gel may help with obtaining adequate images at the suprasternal notch. For submandibular views, a curved array transducer may be more appropriate and will provide a wider field of view of the many structures being imaged at that level.
Thyroid Cartilage
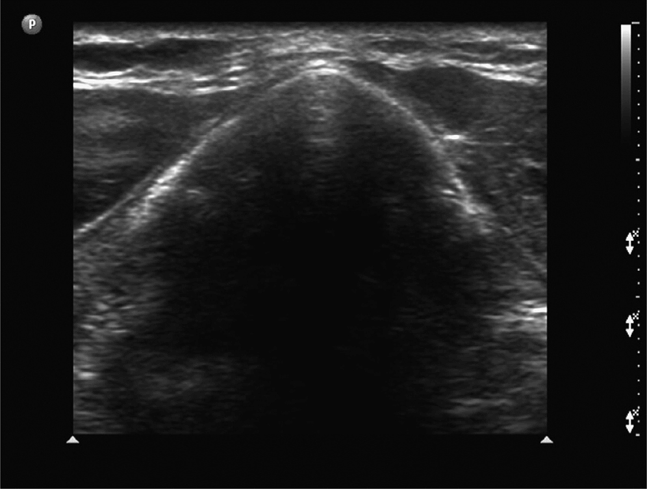
The thyroid cartilage is best imaged in a transverse scan plane in the upper neck with the neck slightly extended. Beneath the skin and subcutaneous tissues the thyroid cartilage appears as an inverted V-shaped structure that exhibits a variety of echogenic appearances, ranging from nearly isoechoic in younger patients to hyperechoic if the cartilage has become calcified. The hypoechoic strap muscles are seen overlying the laminae on each side of the thyroid cartilage. When the thyroid cartilage is hyperechoic, the region beneath will appear nearly anechoic (Figure 18-13) and when the thyroid cartilage is isoechoic with the surrounding muscles, the laryngeal structures beneath (arytenoids, vocal folds, and vocal cords) can frequently be identified (Figure 18-14). When scanning in cross section just above the superior thyroid notch, the anterior portion of the inverted V appears hypoechoic; a thin echogenic line corresponding to the anterior portion of the thyrohyoid ligament may be noted (Figure 18-15). If the transducer is placed in long axis in the midline at the upper portion of the thyroid cartilage, the hyoid bone will be seen in cross section and its prominent posterior acoustic shadow will be noted (Figure 18-16)
Figure 18-13. Short-axis sonogram of the thyroid cartilage below the level of the thyroid notch. Beneath a thin layer of skin and subcutaneous tissue, the two laminae of the thyroid cartilage meet in the anterior midline at about a 90° angle and appear as an inverted V-shape. In some subjects, as in this example, the cartilage will appear hyperechoic and the region beneath anechoic. The hypoechoic structures on either side of the cartilage are the strap muscles.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 ABDOMINAL WALL
ABDOMINAL WALL AIRWAY
AIRWAY BONY FRACTURE EVALUATION
BONY FRACTURE EVALUATION FOREIGN BODY LOCALIZATION
FOREIGN BODY LOCALIZATION MUSCULOTENDINOUS APPLICATIONS
MUSCULOTENDINOUS APPLICATIONS SALIVARY GLANDS
SALIVARY GLANDS MAXILLARY SINUSITIS
MAXILLARY SINUSITIS SKIN AND SOFT TISSUE INFECTIONS
SKIN AND SOFT TISSUE INFECTIONS