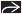Mycobacterium Avium Complex
Jud W. Gurney, MD, FACR
Key Facts
Terminology
NTM pulmonary infection, most commonly caused by MAC, causes 5 types of disease
Classic infection that mimics Mycobacterium tuberculosis
Upper lobe fibrocavitary disease
Solitary or multiple nodules
Bronchiectasis & nodules (Lady Windermere syndrome)
Bronchiectasis, RML, and lingula
Centrilobular nodules
Disseminated disease in immunosuppressed patients
Normal to aggressive cavitary disease
Lymphadenopathy or hepatosplenomegaly
Hypersensitivity pneumonia (“hot tub” lung)
Subacute hypersensitivity pneumonitis pattern
Consolidative pattern associated with achalasia
Pattern of aspiration pneumonia
Imaging Findings
Progression slow (average of 6 years to demonstrate progression)
Top Differential Diagnoses
Post-Primary Tuberculosis
Pathology
Pathophysiology of Lady Windermere syndrome
Hypothesis: Fastidious suppression of cough leads to retained secretions in dependent portions of ventral lung
“Hot tub” lung: Either hypersensitivity reaction to MAC or true infection, preponderance of evidence suggests hypersensitivity reaction
TERMINOLOGY
Abbreviations and Synonyms
Mycobacterium avium intracellulare complex (MAC), nontuberculous mycobacteria (NTM), atypical mycobacteria, chronic obstructive lung disease (COPD)
Definitions
NTM pulmonary infection, most commonly caused by MAC
Other NTM: M. kansasii, M. xenopi, M. fortuitum, M. scrofulaceum, M. gordonae, M. abscessus, and M. chelonae
5 types of disease
Classic infection that mimics Mycobacterium tuberculosis (MTb)
Bronchiectasis & nodules (Lady Windermere syndrome)
Disseminated disease in immunosuppressed patients
Hypersensitivity pneumonia (“hot tub” lung)
Consolidative pattern associated with achalasia and other esophageal motility disorders
IMAGING FINDINGS
CT Findings
Classic infection, 2 patterns, MTb or nodule(s)
Indistinguishable from M. tuberculosis
Upper lobe predominance, apical posterior segments (anterior segment less common)
Thin-walled cavities (thick-walled cavities are less common) hallmark of infection, associated with airspace opacities, masses, and nodules
Mean diameter of cavity is 2.5 cm
Bronchogenic spread with 5-15 mm peripheral centrilobular nodules in dependent lung
Bronchial wall thickening → bronchiectasis
Feeding bronchus: Patent bronchus running into cavitary lesion
Lymphadenopathy and effusions, uncommon
Nodule(s)
Solitary or multiple nodules; solitary nodules mimic bronchogenic carcinoma, range in size from 1 to 5 cm in diameter
Nodules of similar size often cluster together
Associated findings from underlying predisposing factors such as emphysema and pulmonary fibrosis
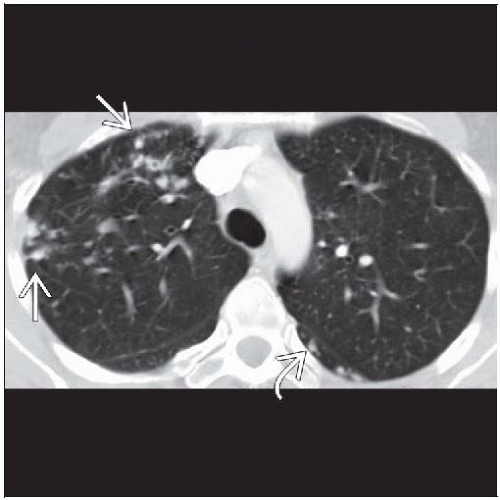
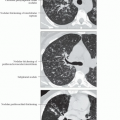
Bronchiectasis & nodules (Lady Windermere syndrome)
Bronchiectasis, cylindrical, distributed mainly in ventral lung, primarily right middle lobe (RML) and lingula
33% of patients with bronchiectasis and nodules will have NTM infection
> 5 lobes with bronchiectasis highly associated with NTM
Centrilobular nodules
85% < 5 mm diameter
80% within same lobe as bronchiectasis
Bilateral multifocal bronchiolitis manifested by
Mosaic pattern of perfusion or hyperinflation with airway narrowing
Tree-in-bud pattern: Branching centrilobular nodules that occur within lobes affected with bronchiectasis but also scattered throughout uninvolved lung
Consolidation, lobular
Cavitation, mediastinal adenopathy, atelectasis rare
Disseminated disease in immunosuppressed patients
Wide range of abnormalities
Often normal or subtle pulmonary findings such as a few scattered centrilobular nodules
Aggressive cavitary disease also possible
Generalized lymphadenopathy or hepatosplenomegaly common
Lymphadenopathy may have low-density necrotic centers
Hypersensitivity pneumonia (“hot tub” lung)
Indistinguishable from other causes of subacute hypersensitivity pneumonitis
Diffuse centrilobular micronodules (85%) usually involving > 40% of lung
Centrilobular nodules either solid (50%) or ground-glass (50%)
Ground-glass opacities (75%), mosaic attenuation, and air-trapping at expiratory scanning (100%)
Achalasia and esophageal motility disorders
Pattern of aspiration pneumonia
Large bilateral dependent areas of consolidation
Pleural effusions (20%)
Cavitary disease (15%)
Radiographic Findings
Radiography
Classic infection
Should suspect in any patient with unexplained upper lung zone opacities
Tubular tram-track opacities suggest underlying bronchiectasis
Apical pleural thickening (50%) often striking; associated with cavitary disease even though cavities may not be radiographically apparent
Progression slow (average of 6 years to demonstrate progression)
Bronchiectasis & nodules (Lady Windermere syndrome)
Should suspect in patients (especially elderly females) with tubular or ill-defined opacities in RML or lingula
Often hyperinflated (but not from emphysema)
Disseminated disease in immunosuppressed patients
Normal radiograph with positive sputum cultures, common (20%)
Abnormal radiographs varied and nonspecific: Small scattered alveolar and nodular opacities, miliary nodules, & mass-like lesions
Cavitation more common in non-AIDS immunosuppressed patients
Hilar or mediastinal lymphadenopathy common and may be only finding
Hypersensitivity pneumonia
Normal radiograph (20%)
Nonspecific diffuse interstitial or nodular ground-glass opacities
Achalasia and esophageal motility disorder
Dilated esophagus, absent stomach bubble
Dependent airspace opacities
DIFFERENTIAL DIAGNOSIS
Post-Primary Tuberculosis
Identical radiologic appearance, cavities generally slightly larger
Unlike NTM, human-to-human transmission
Distinguished by microbiologic features
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree