Clinical Presentation
The patient is a 33-year-old female with a 13-year history of recurrent events involving the nervous system. Initially, she noticed that her feet were painful on first arising in the morning. She had difficulty walking because of the intense discomfort that largely involved the soles of her feet. These symptoms abated spontaneously. Her next neurologic event occurred 4 years later, when she experienced an acute onset of weakness with difficulty in walking. Steroid therapy was introduced and these symptoms abated. On one occasion, she experienced transient double vision and has more recently noticed the onset of blurred vision in the left eye. The motor symptoms have involved all extremities. The most recent event occurred earlier this year. This was characterized by the acute onset of discomfort in the right upper extremity. Her arm became sensitive to sensory stimuli, and she has since been troubled by a feeling of burning, largely involving the right arm and also, to some extent, the lower limbs. Bladder function has deteriorated to the point where she is subject to marked urgency and frequency of micturition and is often incontinent of urine.
Imaging Presentation
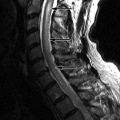
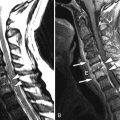
Sagittal T2 and post-contrast, T1-weighted images reveal a long segment of patchy increased T2 signal intensity and enhancement in an enlarged cervical spinal cord from C2-C6 ( Fig. 49-1 ) .

Discussion
Neuromyelitis optica (NMO), also known as Devic’s disease , is an inflammatory disease of the central nervous system that mainly affects, as the name implies, the spinal cord and the optic nerves. NMO has frequently been thought of as a variant of multiple sclerosis (MS); however, clinical, radiologic, laboratory, and pathologic features and treatment have demonstrated that NMO is a distinct entity. NMO can be monophasic or relapsing. Clinically, patients with relapsing NMO are similar to those with MS. However, patients with NMO have more severe attacks than those with MS. Radiologically, spinal cord lesions of NMO are located centrally within the spinal cord and extend over three or more vertebral segments. Atrophy and central cavitation can be seen in later stages of the disease. Patients with MS have more discrete lesions in the dorsolateral aspect of the spinal cord that are usually no longer than a single vertebral segment. Patients with NMO are seropositive for NMO-immunoglobulin G (NMO-IgG), which is rarely seen with MS.
NMO affects young adults, but has been reported from infancy through the ninth decade. The mean age at onset is 29 years (range 1-54 years) for the monophasic type and 39 years (range 6-72 years) for the relapsing type. The ratio of women to men ranges from 1.4 to 1.8:1. NMO appears to be more common in African-Americans, Japanese, and other Pacific Islanders. Demyelinating disease in Asia and India is typically NMO.
Clinically, a viral prodrome precedes the onset of NMO in 30% to 50% of cases. This prodrome may consist of headache, fever, fatigue, respiratory, and gastrointestinal complaints. This may suggest that, at least in some cases, NMO is caused or triggered by an infectious agent. Spinal cord symptoms of NMO worsen over several hours to days and involve motor, sensory, and sphincter function. This may be associated with deep or radicular pain, lower extremity paresthesias and/or weakness. This weakness can progress to complete loss of sensation caudal to the spinal cord lesion. Myelitis in the cervical spine could lead to respiratory failure and death. Optic neuritis in NMO may be uni- or bilateral and can be associated with retro-orbital pain. During a patient’s first episode of NMO, 40% of eyes affected by optic neuritis become completely blind. Most patients have some improvement in vision over time.
Newly proposed diagnostic criteria for NMO require the presence of optic neuritis and acute myelitis along with at least two of three supporting criteria: (1) a region of abnormal T2 signal intensity within the spinal cord extending over three or more vertebral segments, (2) brain magnetic resonance (MR) imaging not having the typical appearance of MS, and (3) NMO-IgG seropositivity. It has been suggested to further subdivide NMO into partial or complete. A complete NMO would have involvement of the optic nerves and spinal cord either simultaneously or consecutively and positive NMO-IgG. A partial NMO would have involvement of either the optic nerves or spinal cord and positive NMO-IgG.
NMO is only one of many etiologies that affect the spinal cord and cause myelopathy. Myelopathy is defined as a neurologic deficit related to the spinal cord. Myelopathy is most commonly caused by compression of the spinal cord either from an osteophyte or disc herniation in the cervical spine and less commonly in the thoracic spine. Extrinsic compression of the spinal cord from trauma, metastatic disease, and primary neoplasm is the next most common cause of myelopathy. Other disease processes such as inflammatory, infectious, autoimmune, idiopathic, neoplastic, vascular, and nutritional disorders can effect the spinal cord directly, although they are more rare.
It is usually not a diagnostic dilemma in identifying causes of myelopathy extrinsic to the spinal cord versus intrinsic to the spinal cord. Computed tomography (CT) or, more preferred, magnetic resonance (MR) imaging readily demonstrates pathologic processes related to the vertebra or intervertebral discs and their relationship to the spinal cord. A history of recent trauma is also important information when evaluating an abnormality of the spinal cord. What is more challenging is narrowing the differential diagnosis of a patient presenting with myelopathic symptoms and an MR image demonstrating nonspecific increased T2 signal intensity in the spinal cord.
Clinically, myelopathy can be subdivided into their methods of presentation. When a patient presents with myelopathy associated with radicular pain, degenerative spondylosis from osteophyte and/or disc herniation, tumor and infection should be suspected. When myelopathy progresses in a stepwise fashion or has a sudden onset, then vascular processes such as vascular malformations, spinal cord infarction. and epidural hematoma should be considered. If myelopathy is painless and slowly progressive, differential considerations should include neoplasm, demyelinating disease, degenerative disease, and nutritional deficiency.
Imaging Features
During an acute event of NMO, there is often a region of increased T2 signal intensity in the central spinal cord that extends over a length of at least three vertebral segments. There may be nodular, patchy, or diffuse enhancement of the spinal cord, sometimes having a mass-like appearance (see Fig. 49-1 ). In the more chronic stages, the spinal cord may demonstrate atrophy and/or cavitation ( Fig. 49-2 ) . Increased T2 signal intensity lesions can be seen within one or both optic nerves. Regions of increased T2 signal intensity can be seen within the brain, most of which are not in the typical periventricular location or have the typical punched-out or flame-shaped configuration of MS lesions.

Differential Diagnosis
Magnetic resonance imaging is the only method of imaging able to evaluate abnormalities intrinsic to the spinal cord. Myelopathy related to an intrinsic spinal cord process has similar imaging characteristics of increased T2 signal intensity, variable enhancement, and/or cord swelling. The location of these signal abnormalities is important in narrowing the differential diagnosis. The spinal cord syndromes can be subdivided into broad categories of those that involve the whole cord, central cord, anterior cord, or posterior cord. The major etiologies causing abnormal spinal cord T2 signal intensity are discussed separately below.
Whole Cross-Sectional Spinal Cord Abnormality
- 1
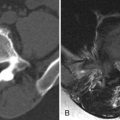
Transverse myelitis is the classic spinal abnormality that involves most or all of a portion of the spinal cord. Transverse myelitis is not a single process but rather a syndrome with multiple causes. Transverse myelitis is associated with viral infections, vaccinations, autoimmune processes, and cancer, although most cases are idiopathic. Transverse myelitis begins with back or radicular pain that quickly leads to bilateral leg paresthesias, an ascending sensory level, paraparesis, and paraplegia. It is most common in middle-aged adults. It is most frequently found in the thoracic spine. The characteristic imaging findings of transverse myelitis are normal size or segmental enlargement of the spinal cord, increased T2 signal intensity involving more than two thirds of the cross-sectional area of the spinal cord, signal abnormality extending over three to four vertebral body levels, and either focal nodular enhancement or some enhancement at the periphery of the spinal cord ( Fig. 49-3 ) .
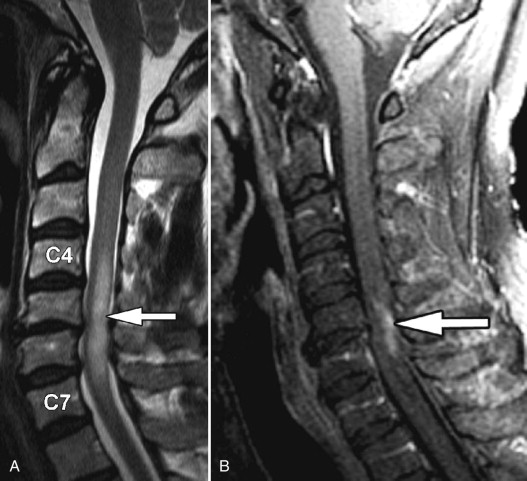
Figure 49-3
Transverse Myelitis.
Sagittal T2-weighted image A demonstrates a long segment region of abnormal increased cord signal intensity ( arrow ) with a normal-sized spinal cord. There is patchy enhancement in the dorsal inferior aspect of this lesion on sagittal post-contrast, T1-weighted image B ( arrow ).
- 2
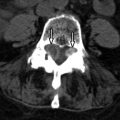
Compressive lesions from spondylosis, large disc herniation, and neoplasm can affect a segment of the entire spinal cord. The etiology of the myelopathy becomes obvious with imaging because MR readily demonstrates a large disc herniation, osteophyte, or primary bone abnormality compressing the spinal cord. Compression can cause increased T2 signal intensity throughout the affected segment of the spinal cord ( Fig. 49-4 ) , and in the subacute phase, may demonstrate some patchy enhancement. The signal abnormality is typically confined to the level of compression. Without decompressive treatment, there can be infarction of the spinal cord at the level of abnormality often seen as two foci of increased T2 signal intensity on either side of the spinal cord (“snake eyes”) compatible with regions of myelomalacia ( Fig. 49-5 ) .

Figure 49-4
Pathologic Fracture with Cord Compression.
Segmental subtle increased spinal cord T2 signal intensity ( arrow ) caused by compression from a pathologic fracture with retropulsion of pathologic bone ( M ).

Figure 49-5
Myelomalacia from Chronic Compression.
Focal regions of increased T2 signal intensity in the central gray matter of the spinal cord on both sides of midline ( arrows ) can be a late sequela of chronic cord compression with ensuing cord myelomalacia.
- 3
Radiation myelopathy causes injury to the white matter of the spinal cord. Patients usually present 9 to 15 months after the end of radiation. Clinically, the patient can present early on with paresthesia (particularly with an inability to perceive pain and temperature). As it progresses, the patient can experience various symptoms including gait abnormalities and hemiplegia. Several factors influence the development of radiation myelopathy including the total delivered dose of radiation, fractionation of the radiation dose (fractionation increases the latent period), the volume of the irradiated tissue (larger volumes decrease the latent period), the linear energy transfer (the greater the transfer the more likely myelopathy is going to occur), and the level of the spinal cord irradiated (posterior and lateral spinal cord involvement in the cervical/upper thoracic region; anterior and lateral spinal cord involvement in the lower thoracic/lumbar region). Three criteria must be satisfied to diagnose radiation myelopathy: (1) the affected spinal cord must be within the radiation field, (2) the neurologic deficit must correspond to the affected spinal cord segment, and (3) metastases or other primary spinal cord lesions must have been excluded. Radiation myelopathy is also best seen with MRI. In the acute phase of radiation myelopathy, the spinal cord is expanded and demonstrates decreased T1 signal and increased T2 signal. Contrast enhancement is variable and may be patchy or ringlike ( Fig. 49-6 ) . In the chronic phase of radiation myelopathy, the affected portions of the spinal cord (often the regions that enhanced) can become atrophic.