Nasal Passages, Mandible, and Upper Airway
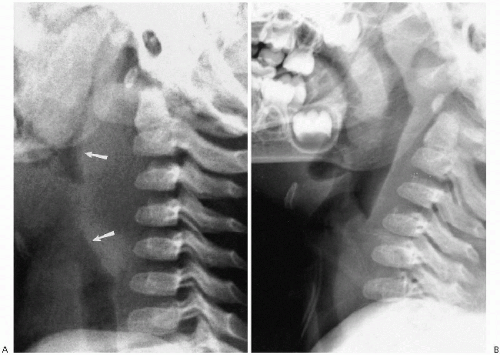
Air in the nose, pharynx, larynx, and upper trachea provides a natural contrast medium that proves most useful in the assessment of the soft tissues of the neck. These tissues are best assessed on the lateral roentgenogram, obtained during inspiration, with the neck partially extended. If the neck is flexed or if the infant is crying (expiration), the airway buckles forward and the prevertebral soft tissues become more prominent, suggesting a mass (Fig. 2.1). Buckling of the airway has been suggested to be the result of upward crowding of the prevertebral soft tissues by the jamming effect of the thymus being pushed into the thoracic inlet during expiration(1). However, this can be avoided if the
examination is properly performed (Fig. 2.1B), whereupon one can clearly identify the entire airway and surrounding soft tissues (Fig. 2.2A). In many cases, the same structures also are visible on frontal projection (Fig. 2.2B), but it is the lateral view that is most uniformly informative. It is for this reason that the plain film study of the airway, especially the lateral view, still is the most important and productive imaging study in the assessment of stridor and other upper airway problems(2). Computed tomography (CT) and magnetic resonance imaging (MRI) are used in specific instances, but initial impressions usually are accomplished on clinical and plain film findings.
examination is properly performed (Fig. 2.1B), whereupon one can clearly identify the entire airway and surrounding soft tissues (Fig. 2.2A). In many cases, the same structures also are visible on frontal projection (Fig. 2.2B), but it is the lateral view that is most uniformly informative. It is for this reason that the plain film study of the airway, especially the lateral view, still is the most important and productive imaging study in the assessment of stridor and other upper airway problems(2). Computed tomography (CT) and magnetic resonance imaging (MRI) are used in specific instances, but initial impressions usually are accomplished on clinical and plain film findings.
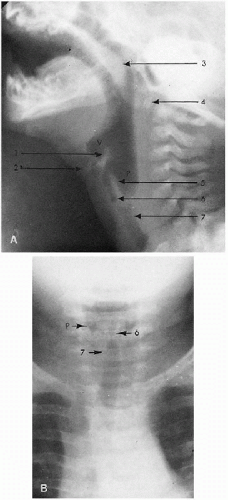
As far as the retropharyngeal soft tissues are concerned, undue thickening should alert one to the presence of a retropharyngeal mass or inflammatory process, but it should be remembered that this area is proportionately thicker in newborn and young infants than in older children because the vertebral bodies are not yet fully ossified. Unfortunately, it is difficult to outline precise prevertebral soft tissue measurements, but it has been generally accepted that in infants, with the neck fully extended, the soft tissue space from C1 to C4 is equal to one-half of the width of the vertebral body. Later on, as the infant grows older, the vertebral bodies become larger and wider, and prevertebral soft tissues become correspondingly less prominent. Below the
level of C4, that is, below the level of the larynx, the soft tissue space normally doubles in width. The resulting step-off between the posterior pharyngeal and tracheal walls is probably the most important thing to note. On a well-positioned film, if the step-off is lost, one should suspect retropharyngeal pathology.
level of C4, that is, below the level of the larynx, the soft tissue space normally doubles in width. The resulting step-off between the posterior pharyngeal and tracheal walls is probably the most important thing to note. On a well-positioned film, if the step-off is lost, one should suspect retropharyngeal pathology.
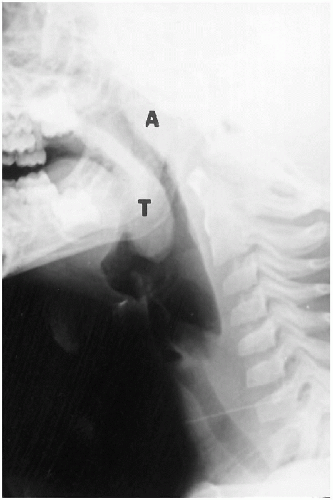 FIGURE 2.3. Normal tonsils and adenoids. Note the prominent tonsils (T) and adenoids (A) in this 3-year-old child. |
TABLE 2.1. NORMAL NASOPHARYNGEAL LYMPHOID TISSUE | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Nasopharyngeal adenoidal tissue is sparse in the newborn, but as the infant grows older, it becomes more abundant (Fig. 2.3). For specific measurements of the adenoids, one is referred to Table 2.1.
REFERENCES
1. Mandell GA, Bellah RD, Boulden MEC, et al. Cervical trachea: dynamics in response to herniation of the normal thymus. Radiology 1993;186:383-386.
2. John SD, Swischuk LE. Stridor and upper airway obstruction in infants and children. Radiographics 1992;12:625-643.
ABNORMALITIES OF THE NASAL PASSAGES
Choanal Atresia
Choanal atresia, a congenital obstruction of the nasal passages, can be either membranous or bony(1). When it is bilateral, severe respiratory distress develops, and, in fact, until the infant learns to mouth-breathe, the mouth may have to be held open mechanically. Feeding also can be a problem. When choanal atresia is unilateral, it poses less of a problem and frequently passes unnoticed until the infant is older.
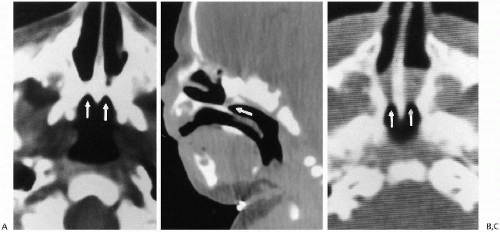
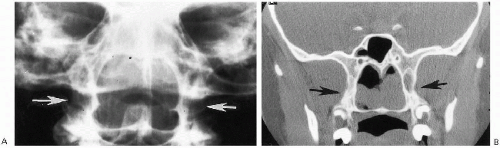
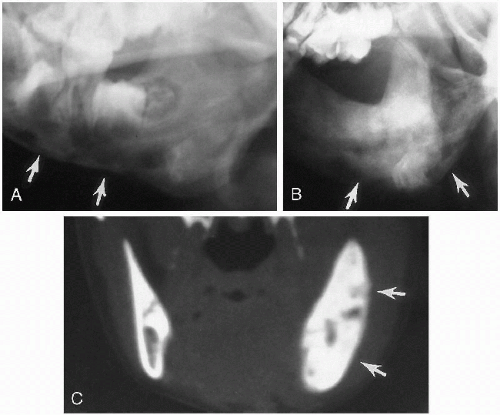
Plain film roentgenographic changes are absent, but in the past, contrast studies of the nasal passages were helpful. These now have been replaced by CT(2), where detailed delineation of the abnormal anatomy is possible. CT identifies both the membranous and the bony forms of the condition (Fig. 2.4). Slovis et al.(2) have indicated that in bony atresia, the vomer (midline bone) is thickened and also that there is inward thickening of the lateral nasal bony wall
(Fig. 2.4A). The membrane in membranous obstructions may be thin, and in addition, there is less thickening of the lateral nasal wall and vomer (Fig. 2.4B). The precise location of the obstructing membrane or bone can be further delineated with sagittal reconstruction views (Fig. 2.4B). Associated mucoid impaction can appear as an intranasal mass(3) and on fluid collections may suggest that an obstructing membrane is thicker than it really is (Fig. 2.4C). To remedy this, it has been suggested that the nasal passages be thoroughly cleaned and that topical decongestants be applied before CT examination is undertaken(2).
(Fig. 2.4A). The membrane in membranous obstructions may be thin, and in addition, there is less thickening of the lateral nasal wall and vomer (Fig. 2.4B). The precise location of the obstructing membrane or bone can be further delineated with sagittal reconstruction views (Fig. 2.4B). Associated mucoid impaction can appear as an intranasal mass(3) and on fluid collections may suggest that an obstructing membrane is thicker than it really is (Fig. 2.4C). To remedy this, it has been suggested that the nasal passages be thoroughly cleaned and that topical decongestants be applied before CT examination is undertaken(2).
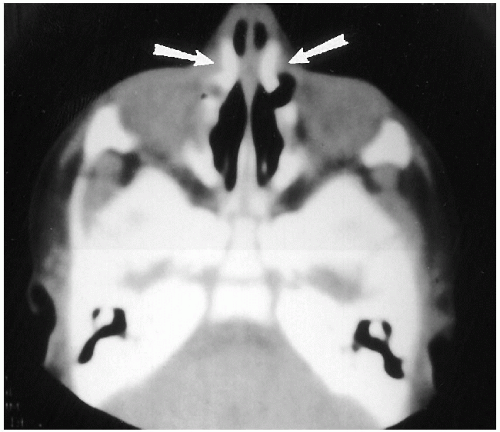
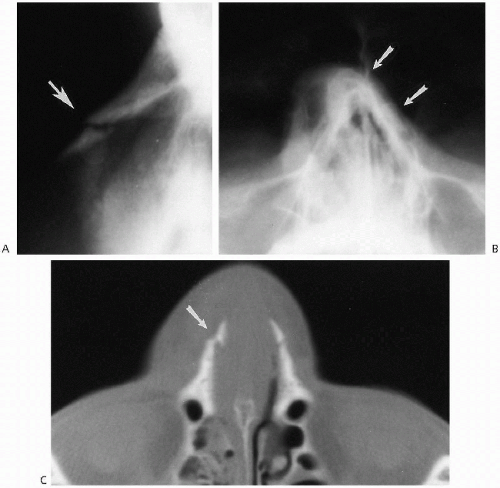
The vast majority of congenital stenoses or frank obstructions of the nasal passages occur posteriorly, in the form of posterior choanal stenosis or atresia. Rarely, somewhat similar stenoses can be encountered anteriorly and have been termed “inlet”(4), or congenital, nasal piriform aperture stenosis(5,6,7). This abnormality also is best demonstrated with CT (Fig. 2.5), where it has been suggested that a width of less than 11 mm through the aperture be considered abnormal(6). These patients have been demonstrated to have associated abnormalities consisting of absence of the anterior pituitary gland and abnormal dentition(6).
REFERENCES
1. Stahl RS, Jurkiewicz MJ. Congenital posterior choanal atresia. Pediatrics 1985;76:429-436.
2. Slovis TL, Renfro B, Watts FB, et al. Choanal atresia: precise CT evaluation. Radiology 1985;155:345-348.
3. Kleinmann P, Winchester PP. Pseudotumor of the nasal fossa secondary to mucoid impaction in choanal atresia. Pediatr Radiol 1975;4:47-48.
4. Ey EH, Han BK, Towbin RB, et al. Bony inlet stenosis as a cause of nasal airway obstruction. Radiology 1988;168:477-479.
5. Bignault A, Castillo M. Congenital nasal piriform aperture stenosis. AJNR 1994;15:877-878.
6. Belden CJ, Mancuso AA, Schmalfuss IM. CT features of congenital nasal piriform aperture stenosis: initial experience. Radiology 1999;213:495-501.
7. Beregszaszi M, Leger J, Gaerl C, et al. Nasal piriform aperture stenosis and absence of the anterior pituitary gland: report of two cases. J Pediatr 1996;128:858-861.
Nasal Polyps
Nasal polyps are not particularly common in children. Occasionally so-called “allergic polyps” are encountered, and occasionally recurrent bouts of allergy and infection lead to chronic hypertrophic polypoid rhinosinusitis. However, most commonly when nasal polyps are encountered in children, the underlying problem is cystic fibrosis. In any of
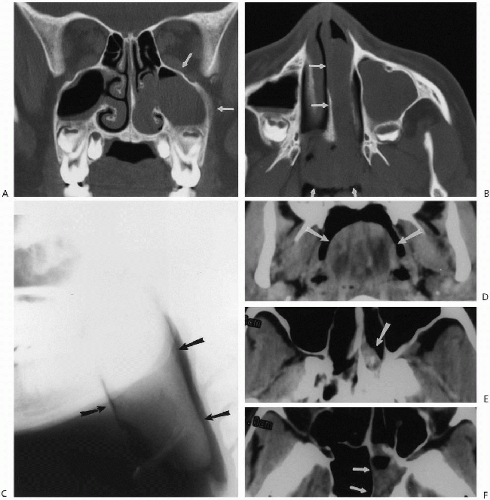
these cases, if the polyps are massive and chronic, they can completely fill the nose, extend into the nasopharynx, and even widen the nose. If clearer definition of polyps is required, CT examination is useful (Fig. 2.6).
these cases, if the polyps are massive and chronic, they can completely fill the nose, extend into the nasopharynx, and even widen the nose. If clearer definition of polyps is required, CT examination is useful (Fig. 2.6).
Antrochoanal polyps are peculiar lesions of older children and young adults(1,2,3). These benign polyps are of unknown etiology but probably not inflammatory or allergic in origin. They most commonly arise from the maxillary sinuses but also can arise from the sphenoid and ethmoid sinus cavities(4,5,6,7). In any case, they may fill the nasal cavity or plunge back into the oropharynx. As such, they can present as a pharyngeal mass (Fig. 2.7). In addition, the
involved sinus, usually the ipsilateral maxillary sinus, is opacified (Fig. 2.7, A and B). Ultimately, it is the CT study that more clearly demonstrates the polyp and its site of origin (Fig. 2.7). Antrochoanal polyps can produce secondary symptoms such as sleep apnea(8) and dysphagia(6). Finally, it should be noted that the so-called “hairy” polyp can be encountered in the newborn(9).
involved sinus, usually the ipsilateral maxillary sinus, is opacified (Fig. 2.7, A and B). Ultimately, it is the CT study that more clearly demonstrates the polyp and its site of origin (Fig. 2.7). Antrochoanal polyps can produce secondary symptoms such as sleep apnea(8) and dysphagia(6). Finally, it should be noted that the so-called “hairy” polyp can be encountered in the newborn(9).
REFERENCES
1. Min Y-G, Chung JW, Shin J-S, et al. Histologic structure of antrochoanal polyps. Acta Otolaryngol 1995;115:543-547.
2. Rashid A-MH, Soosay G, Morgan D. Unusual presentation of a nasal antrochoanal polyp. Br J Clin Pract 1994;48:108-109.
3. Sharma HS, Daud ARA. Antrochoanal polyp—a rare paediatric emergency. Int J Pediatr Otorhinolaryngol 1997;41:65-70.
4. Crampette L, Mondain M, Rombaux PH. Sphenochoanal polyp in children; diagnosis and treatment. Rhinology 1995;33:43-45.
5. Weissman JL, Tabor EK, Curtin HD. Sphenochoanal polyps: evaluation with CT and MR imaging. Radiology 1991;178:145-148.
6. Lopatin A, Bykova V, Piskunov G. Choanal polyps: one entity, one surgical approach? Rhinology 1997;35:79-83.
7. Swischuk LE, Hendrick EP. Antrochoanal polyp originating from sphenoid sinus causing acute dysphagia. Emerg Radiol 2000;7: 358-360.
8. Rodgers GK, Chan KH, Dahl RE. Antral choanal polyp presenting as obstructive sleep apnea syndrome. Arch Otolaryngol Head Neck Surg 1991;117:914-916.
9. Kelly A, Bough ID Jr, Luft JD, et al. Hairy polyp of the oropharynx: case report and literature review. J Pediatr Surg 1996;31: 704-705.
Nasal Foreign Bodies
Nasal Trauma
Nasal trauma is common in infants but most of the time does not lead to underlying bony injury. However, fractures can occur transversely across the nasal bone or longitudinally in the septal portion of the bone. What is most important is to determine whether septal deviation has occurred, and this is best accomplished on the Water’s view or with CT (Fig. 2.8). It is important not to misinterpret the normal nasociliary groove and frontonasal suture for fractures.
Nasal Tumors and Cysts
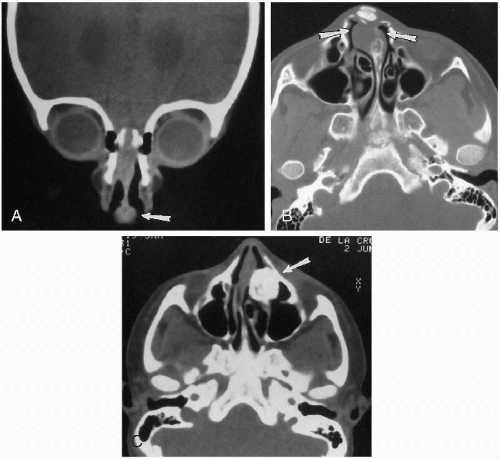
Nasal tumors and cysts are not particularly common, and as far as cysts are concerned, dermoid cysts are probably the most common(1). Most often, they are midline, round, and best demonstrated with CT or MRI(2) (Fig. 2.9, A and B). Patients with nasal dermoids also may demonstrate hypertelorism, and the findings should be differentiated from those seen with nasal encephalocele and congenital dermal sinus. In both the latter instances, there is communication with the subarachnoid space of the brain, while with a dermoid cyst, no such communication exists. Nasal encephaloceles are discussed in Chapter 6.
Nasal tumors generally are uncommon in childhood, but include nasal gliomas(1), teratomas, fibrous histiocytomas(2), osteomas, olfactory neuroblastomas(3), rhinolithiasis(4), and hemangiomas. Nasal gliomas present with a progressively enlarging mass over the bridge of the nose. Hypertelorism and localized bony erosion can be seen, and some of these tumors may communicate intracranially. Teratomas can be identified by the presence of extensive calcification, and osteomas, of course, are very dense and radiopaque (Fig. 2.9C). All of these lesions finally are imaged with CT and/or MRI.
REFERENCES
1. Barkovich AJ, Vandermarck P, Edwards MSB, et al. Congenital nasal masses: CT and MR imaging features in 16 cases. AJR 1991;156:587-598.
2. Shearer WT, Schreiner RL, Ward SP, et al. Benign nasal tumor appearing as neonatal respiratory distress; first reported case of nasopharyngeal fibrous histiocytoma. Am J Dis Child 1973; 126:238-241.
3. Ferlito A, Micheau C. Infantile olfactory neuroblastoma: a clinicopathological study with review of the literature. J Otorhinolaryngol 1971;41:40–45.
4. Royal SA, Gardner RE. Rhinolithiasis: an unusual pediatric nasal mass. Pediatr Radiol 1998;28:54-5.
ABNORMALITIES OF THE TONGUE
A large tongue can be seen with acute glossitis(1) and primary tumors such as rhabdomyosarcoma(2), lymphangioma(3), and hemangioma. Enlargement also can be seen with cretinism and the Beckwith-Wiedemann syndrome. A small tongue is less common and often is associated with a small mandible. An absent tongue can be seen as part of the aglossia-adactylia syndrome(4). Finally, glossoptosis (posterior dropping of the tongue) can lead to sleep apnea and can be documented fluoroscopically during sleep(5).
REFERENCES
1. Stoddard JJ, Deshpande JK. Acute glossitis and bacteremia caused by Streptococcus pneumoniae: case report and review. Am J Dis Child 1991;145:598-599.
2. Liebert PS, Stool SE. Rhabdomyosarcoma of the tongue in an infant. Results of combined radiation and chemotherapy. Ann Surg 1973;178:621.
3. Lobitz B, Lang T. Lymphangioma of the tongue. Pediatr Emerg Care 1995;11:1983-1185.
4. Johnson GF, Robinow M. Aglossia-adactylia. Radiology 1978; 128:127-132.
5. Donnelly LF Strife JL, Myer CM III. Glossoptosis (posterior displacement of the tongue during sleep): a frequent cause of sleep apnea in pediatric patients referred for dynamic sleep fluoroscopy. AJR 2000;175:1557-1559.
ABNORMALITIES OF THE SALIVARY GLANDS
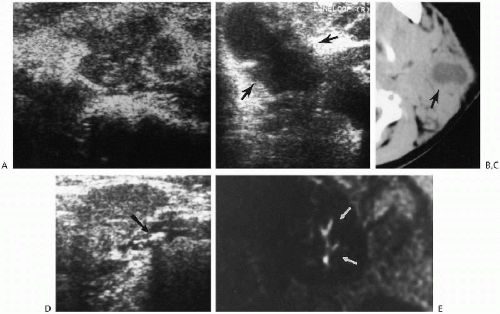
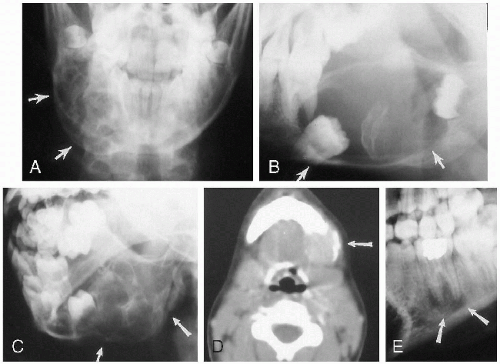
Infections of the salivary glands usually are viral and most often are caused by the mumps virus. Suppurative infections usually result from bacterial infection. Some children also have chronic recurrent swelling of the salivary glands of unknown etiology, but presumed to be of autoimmune origin(1). Sjöogren’s syndrome, also an autoimmune-mediated disease, is uncommonly documented in children, as are salivary gland stones. The stones may be visualized on plain films, CT, or with ultrasound and can be located in the ducts or in the glands themselves. In the past, the ducts themselves were visualized by way of contrast sialography, but this has been replaced by MR sialography(2) (Fig 2.10D).
Generally, lesions of the salivary glands now are first investigated with ultrasound(3, 4) and then CT or MR. Ultrasound can detect stones, abscesses, and intraglandular lymphoid tissue (Fig. 2.10, A-E). The latter, first believed to be seen only in human immunodeficiency virus-positive patients(5, 6), is now known also to occur in other patients. Ultrasonographically, infected salivary glands show a nonspecific pattern of echogenicity of the enlarged gland (Fig. 2.10A). If suppuration occurs, a central hypoechoic collection of pus can be seen (Fig. 2.10B ).
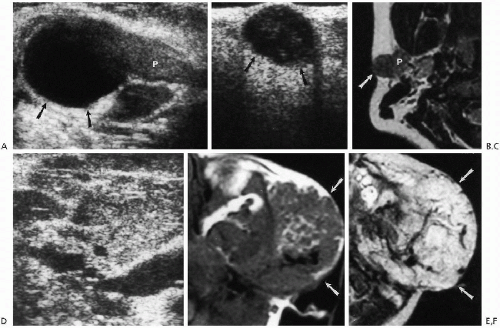
Salivary gland stones usually are the result of chronic infection but also can be seen with cystic fibrosis. Tumors of the salivary glands in childhood, be they primary or secondary, are uncommon but include mixed tumors, carcinomas(7), embryonomas(8), teratomas(9), lipomas(10), mucoepidermoid tumors, and hemangiomas(11). For the most part, it is difficult to histologically type the tumor on the basis of the features seen on any given imaging modality (Fig. 2.11), except for hemangiomas. Hemangiomas produce coarse echogenicity on ultrasound and markedly
increased signal on T2-weighted MR images. In addition, with both studies, one can identify feeding vessels and internal blood flow. This is especially well documented with color flow Doppler.
increased signal on T2-weighted MR images. In addition, with both studies, one can identify feeding vessels and internal blood flow. This is especially well documented with color flow Doppler.
REFERENCES
1. Wilson WR, Eavey RD, Lang DW. Recurrent parotitis during childhood. Clin Pediatr 1980;19:235-236.
2. Becker M, Marchal F, Becker CD, et al. Sialolithiasis and salivary ductal stenosis: diagnostic accuracy of MR sialography with a three-dimensional extended-phase conjugate-symmetry rapid spin-echo sequence. Radiology 2000;217:347-358.
3. Seibert RW, Seibert JJ. High-resolution ultrasonography of the parotid gland in children. Part II. Pediatr Radiol 1988;19:13-18.
4. Garcia CJ, Flores PA, Arce JD, et al. Ultrasonography in the study of salivary gland lesions in children. Pediatr Radiol 1998; 28:418-425.
5. Goddart D, Francois A, Ninane J, et al. Parotid gland abnormality found in children seropositive for the human immunodeficiency virus (HIV). Pediatr Radiol 1990;20:355-357.
6. Soberman N, Leonidas JC, Berdon WE, et al. Parotid enlargement in children seropositive for human immunodeficiency virus: imaging findings. AJR 1991;157:553-556.
7. Rogers DA, Ral BN, Bowman L, et al. Primary malignancy of the salivary gland in children. J Pediatr Surg 1994;29:44-47.
8. Som PM, Brandwein M, Silvers AR, et al. Sialoblastoma (embryoma): MR findings of a rare pediatric salivary gland tumor. AJNR 1997;18:847-850.
9. Rose PE, Howard ER. Congenital teratoma of the submandibular gland. J Pediatr Surg 1982;17:414-416.
10. Holland AJA, Hay GSB, Brennan BA. Parotid lipomatosis. J Pediatr Surg 1996;31:1422-1423.
11. Hebert G, Ouimet-Oliva D, Ladouceur J. Vascular tumors of the salivary glands in children. AJR 1975;123:815-819.
ABNORMALITIES OF THE MANDIBLE
Hypoplasia of the Mandible (Micrognathia)
Hypoplasia of the mandible can involve the entire mandible or just one side. When unilateral underdevelopment is present,
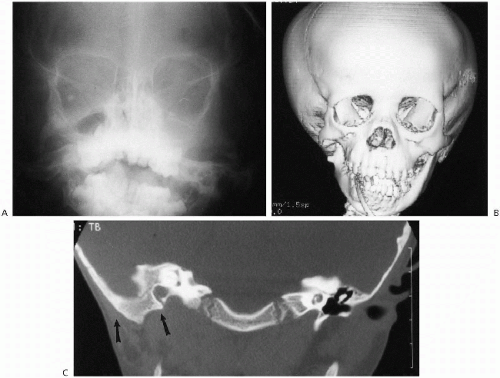
the condition frequently is referred to as “hemifacial microsomia.” In such cases, associated temporomandibular joint hypoplasia, facial hypoplasia, and congenital hearing defects can be seen(1). The imaging findings are straightforward and especially well demonstrated with three-dimensional reconstructed CT studies (Fig. 2.12).
the condition frequently is referred to as “hemifacial microsomia.” In such cases, associated temporomandibular joint hypoplasia, facial hypoplasia, and congenital hearing defects can be seen(1). The imaging findings are straightforward and especially well demonstrated with three-dimensional reconstructed CT studies (Fig. 2.12).
Generalized hypoplasia of the mandible frequently is seen as part of the “first arch” syndromes (e.g., Pierre Robin syndrome, Goldenhar’s syndrome, Weyers’ mandibulofacial dysostosis, and Treacher Collins mandibulofacial dysostosis). It is also often seen in the trisomy 17-18 and 13-15 and cri-du-chat syndromes. In all cases, there is mandibular underbiting and recessing of the mandible and tongue into the oropharynx (Fig. 2.13A). This often leads to severe airway compromise and marked respiratory distress. Cor pulmonale with pulmonary edema (secondary to chronic upper airway obstruction) also can result.
As the infant grows older, the mandible usually becomes larger, more normal in position, and less prone to produce airway obstruction. Nonetheless, in the early days and weeks of life, mandibular hypoplasia and its associated posterior displacement of the tongue can produce profound respiratory distress, especially if the infant is kept in a recumbent position. Therefore, these infants should be kept in the prone position, and in severe cases, prolonged nasoesophageal intubation is required.
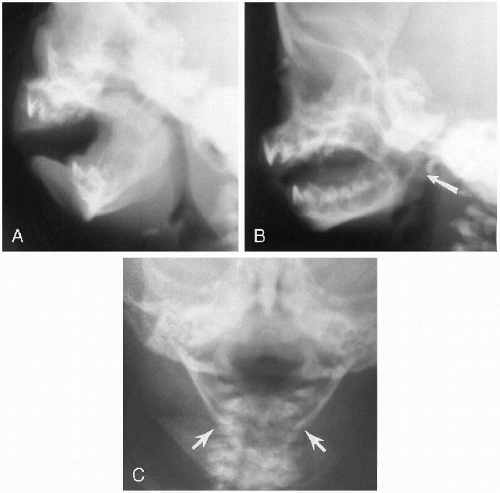
Another variation of mandibular hypoplasia includes isolated hypoplasia of the mandibular rami. In these cases, the body of the mandible is relatively normal, but the rami short (Fig. 2.13B). Still another variation is smallness of the
mandible with a pointed symphysis menti, a deformity usually seen with aglossia (Fig. 2.13C).
mandible with a pointed symphysis menti, a deformity usually seen with aglossia (Fig. 2.13C).
REFERENCE
1. Cavo JW Jr, Pratt LL, Alonso WA. First branchial cleft syndromes and associated congenital hearing loss. Laryngoscope 1976;86: 739-745.
Inflammatory Lesions of the Mandible
Osteomyelitis of the mandible is rarely encountered in the young infant, but later may be seen in association with chronic tooth infection or as a focus of hematogenous osteomyelitis. Although most often osteomyelitis is due to a pyogenic organism, it also can be seen with actinomycosis or other fungal disease. Whatever the cause, the roentgenographic findings are about the same, for there is mandibular destruction, and when the disease is chronic, reactive hyperostosis occurs. A paramandibular mass also may be present and often is best demonstrated with CT or MRI scanning (Fig. 2.14).
In the past, one of the most common inflammatory conditions to involve the mandible of a young infant was infantile cortical hyperostosis. This condition is discussed in more detail in the chapter on bone disease, but the mandible frequently was involved and, indeed, the presenting problem. Usually, there is considerable swelling of the jaw, and roentgenographically, the characteristic findings are those of periosteal new bone deposition along the mandible (Fig. 2.15). This disease, however, now is virtually nonexistent, suggesting that it may have been due to some infectious process, perhaps a viral infection.
The findings should not be confused with reactive periostitis secondary to cellulitis and lymphadenitis of the submandibular lymph glands(1). This condition, usually occurring in older infants and children, has periosteal new bone appear a week or so after the lymph node inflammation begins. The underlying bone is completely normal.
REFERENCE
1. Suydan MJ, Mikity VG. Cellulitis with underlying inflammatory periostitis of the mandible. AJR 1969;56:133-135.
Trauma to the Mandible
For the most part, mandibular fractures are sustained with forceful injuries to the chin, and not mere tumbles. In some cases, only transient problems with temporomandibular joint movement occur, but in other instances, one can encounter a variety of hairline to frankly displaced or
angled fractures of the mandible. Because the mandible is a ring-like bone, fractures in more than one place tend to occur. CT is best for demonstrating mandibular fractures, although Panorex (Gendex) studies also are useful.
angled fractures of the mandible. Because the mandible is a ring-like bone, fractures in more than one place tend to occur. CT is best for demonstrating mandibular fractures, although Panorex (Gendex) studies also are useful.
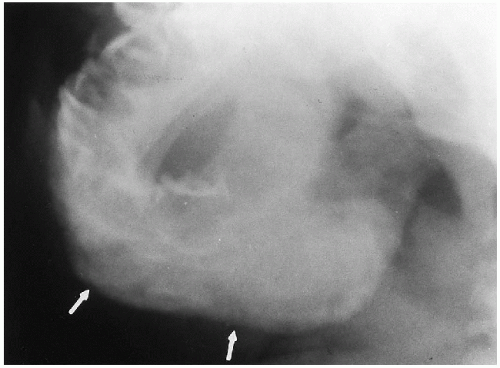 FIGURE 2.15. Caffey’s disease (infantile cortical hyperostosis) of the mandible. Note homogeneous, thick periosteal new bone deposition (arrows) on the mandible. |
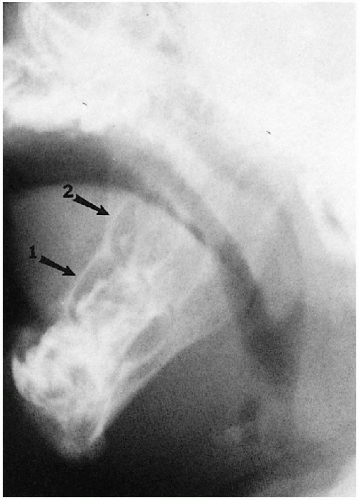
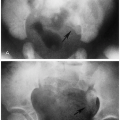
It should be noted that bending fractures of the mandibular condyles also may be seen in infants and young children and, indeed, are common(1) (Fig. 2.16). These fractures are easily overlooked(2) and usually are the result of falling on the chin(3).
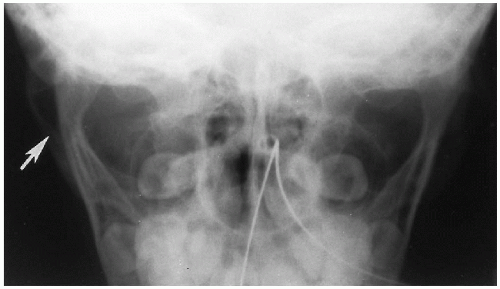 FIGURE 2.16. Bending fracture of mandible. Note the bending fracture of the right mandibular ramus (arrow). |
REFERENCES
1. Ahrendt D, Swischuk LE, Hayden CK Jr. Incomplete (bending?) fractures of the mandibular condyle in children. Pediatr Radiol 1984;14:140-141.
2. Myall RWT, Sandor GKB, Gregory CEB. Are you overlooking fractures of the mandibular condyle? Pediatrics 1987;79:639-641.
3. Hurt TL, Fisher B, Peterson BM, et al. Mandibular fractures in association with chin trauma in pediatric patients. Pediatr Emerg Care 1988;4:121-123.
Temporomandibular Joint
The temporomandibular joint now usually is best assessed with MRI where open and closed views are obtained, but the joints also can be assessed with CT and sagittal reconstruction. In childhood, one may see congenital hypoplasia of the mandible and condyle (see Fig. 2.11B), and while most times the finding is isolated, it also may be associated with some of the syndromes producing generalized mandibular hypoplasia noted in the previous section. Temporomandibular joint dislocation can occur with trauma to the chin, and dislocation also can occur secondary to exaggerated opening of the mouth, as can occur with vomiting(1). The temporomandibular joint also can be involved in rheumatoid arthritis, but joint space narrowing secondary to cartilage destruction is seen with advanced disease only. MR is useful in evaluating the temporomandibular joints in rheumatoid arthritis(2).
REFERENCES
1. Whiteman PJ, Pradel EC. Bilateral temporomandibular joint dislocation in a 10-month-old infant after vomiting. Pediatr Emerg Care 2000;16:418-419.
2. Kuseler A, Pedersen TK, Herlin T, et al. Contrast enhanced magnetic resonance imaging as a method to diagnose early inflammatory changes in the temporomandibular joint in children with juvenile chronic arthritis. J Rheumatol 1998;25:1406-1412.
Tumors and Cysts of the Mandible
Tumors of the mandible are rather uncommon(1) and are extremely rare in the newborn infant. In older children, one may encounter ossifying and nonossifying fibromas, cementomas, ameloblastomas, and fibrous dysplasia. Ossifying fibromas and cementomas usually are unilocular, with distinct margins. They are often seen to contain variable amounts of calcifications within them. Ameloblastomas may produce multilocular, expanding cystic lesions that may be difficult to differentiate from other cystic abnormalities of the mandible.
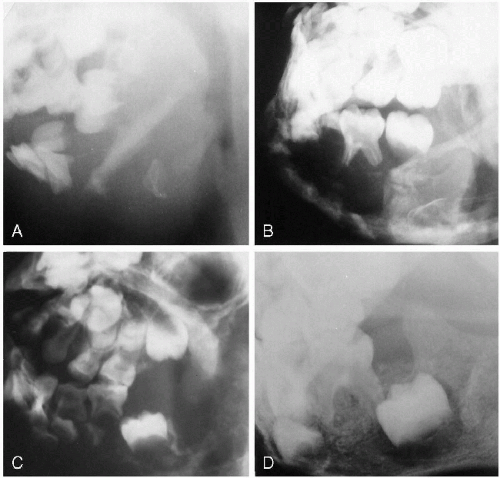
Fibrous dysplasia may involve the mandible as part of generalized fibrous dysplasia but more often appears as a solitary finding. In the mandible, it usually produces a multiloculated cystic lesion and may be unilateral or bilateral (Fig. 2.17A). It tends to be familial, and in such cases, bilateral involvement leads to the term “cherubism”(1, 2).
As far as cysts of the mandible are concerned, the two most commonly occurring ones in childhood are dentigerous cysts and the so-called “reparative” or “giant cell granuloma”
(3, 4). Odontogenic, primordial cysts are rare and develop in the tooth bud before the crown is formed. Dentigerous cysts are much more common and usually present with swelling of the mandible. They demonstrate a typical unilocular appearance with a displaced, ectopic, and tilted tooth in their periphery (Fig. 2.17B). The margins of the cyst are well defined but can be very thin, and occasionally the roots of the involved tooth may be eroded. Dentigerous cysts may be unilateral or bilateral and are common manifestations of the basal cell nevus syndrome.
(3, 4). Odontogenic, primordial cysts are rare and develop in the tooth bud before the crown is formed. Dentigerous cysts are much more common and usually present with swelling of the mandible. They demonstrate a typical unilocular appearance with a displaced, ectopic, and tilted tooth in their periphery (Fig. 2.17B). The margins of the cyst are well defined but can be very thin, and occasionally the roots of the involved tooth may be eroded. Dentigerous cysts may be unilateral or bilateral and are common manifestations of the basal cell nevus syndrome.
Reparative granulomas are a peculiarity of childhood, and their roentgenographic appearance is that of either a unilocular or, more often, a multilocular cystic lesion (Fig. 2.17, C and D). Although originally believed to be developmental, they probably represent a reaction to trauma or inflammatory insult to the mandible. Generally, the lesion is well circumscribed and involves the posterior body and ramus of the mandible.
Finally, a note regarding the so-called “radicular cyst” of the mandible is in order. This is not a true cyst but rather an apical root abscess seen with carious teeth in older children. These lesions seldom become very large, are intimately related to a root tip, and usually are associated with surrounding demineralization and indistinctness of the cyst wall (Fig. 2.17E). The tooth root may be partially resorbed, and caries may be seen on its surface.
Destruction of the mandible also can be seen with leukemia, lymphoma, metastatic disease, (usually neuroblastoma), and histiocytosis X. Histiocytosis X often leads to a very homogeneous and complete destruction of the mandible, leaving the teeth to appear to be floating in a liquid matrix (Fig. 2.18, A and B). This has led to the term “floating teeth sign,” but while very suggestive of histiocytosis X, the sign can be seen with other causes of bony destruction such as metastases to the mandible (Fig. 2.18, C and D) or leukemia.
REFERENCES
1. Kozlowski K, Masel J, Sprague P, et al. Mandibular and paramandibular tumors in children. Report of 16 cases. Pediatr Radiol 1981;11:183-192.
2. Cornelius EA, McClendon JL. Cherubism—hereditary fibrous dysplasia of the jaws. AJR 1969;106:136-140.
3. Waldron CA, Shafer WG. The central giant-cell reparative granuloma of the jaws: an analysis of 38 cases. Am J Clin Pathol 1966;45:437-447.
4. Bodner L, Bar-Ziv J. Radiographic features of central giant cell granuloma of the jaws in children. Pediatr Radiol 1996;26: 148-151.
Dental Abnormalities
In the normal newborn infant, unerupted teeth with well-calcified crowns are present in the mandible and maxilla. In fact, the appearance of the enamel crown of the first and second molars can be used to determine gestational age(1). The roots of the various teeth are usually poorly defined, but the enamel cap and surrounding dental sac (radiolucent space around the tooth) are readily identified (Fig. 2.19).
Underdevelopment or total absence of the teeth can occur with conditions such as the aglossia-adactylia syndrome, cleidocranial dysostosis, pyknodysostosis, ectodermal dysplasia (anhidrotic type), and the otopalatodigital syndrome. The most commonly appreciated of these conditions is ectodermal dysplasia, and the findings can be seen at birth (Fig. 2.20A).
Premature neonatal teeth are even rarer but can be seen with conditions such as the Ellis-van Creveld syndrome, the Hallermann-Streiff syndrome, the adrenogenital syndrome, and the pachyonychia congenita syndrome. In addition, occasionally premature teeth may appear in normal neonates, on both a sporadic(2) and a familial(3) basis. We have also seen premature eruption of natal teeth in an infant whom we believed to have Noonan’s syndrome (Fig. 2.20B). Odontogenesis imperfecta is the term applied to the generally underdeveloped and dysplastic teeth seen with osteogenesis imperfecta.
REFERENCES
1. Kuhns LR, Sherman MP, Poznanski AK. Determination of neonatal maturation on the chest radiography. Radiology 1972;102: 597-603.
2. King NM, Lee AMP. Prematurely erupted teeth in newborn infants. J Pediatr 1989;114:807-809.
3. Sibert JR, Porteous JR. Erupted teeth in the newborn; 6 members in a family. Arch Dis Child 1974;49:492-493.
NECK MASSES
Neck masses are common in infants and children, and one’s best initial imaging modality is ultrasound. Ultrasound can very quickly determine whether the mass is cystic or solid, and thereafter MRI provides excellent soft tissue resolution and geography for location of the mass. Little else in the way of imaging often is required, but if it is, we favor MRI over CT. If the mass is related to the thyroid gland, one also should perform nuclear scintigraphy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree