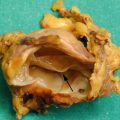
Fig. 1
Insulinoma. (a) Angiography: small hypervascular nodule (arrow) in the body of the pancreas, characterized by the typical capillary blush. (b) Surgical specimen (enucleation): small nodule of 1 cm. (c, d) Histopathology: high cellularity at hematoxylin and eosin staining, and faint positivity at immunohistochemical staining with anti-insulin antibody
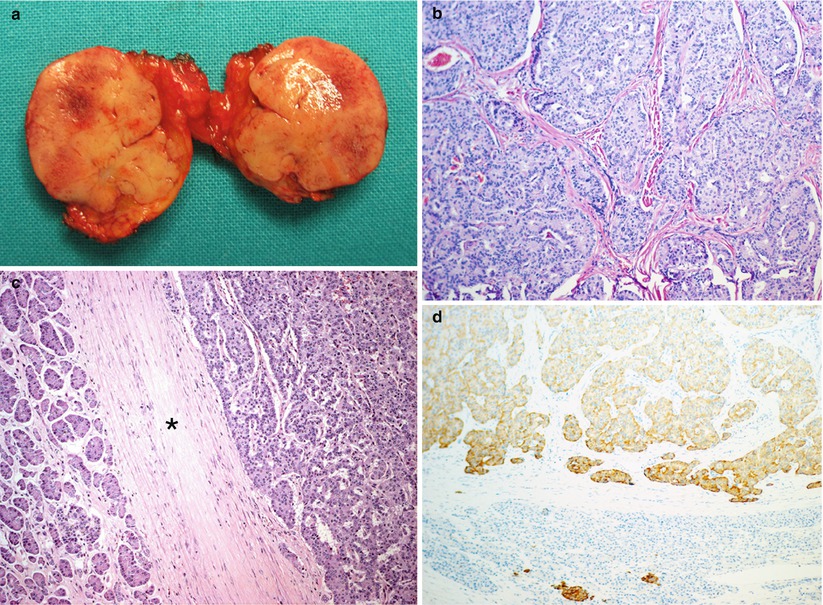
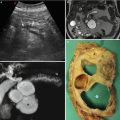
Fig. 2
Insulinoma. (a) Surgical specimen (enucleation): small round capsulated nodule. (b–d) Histopathology: hematoxylin and eosin staining demonstrates high cellularity and the presence of a thick capsule surrounding the lesion (asterisk in c). Positivity at immunohistochemical staining (d) with anti-insulin antibody
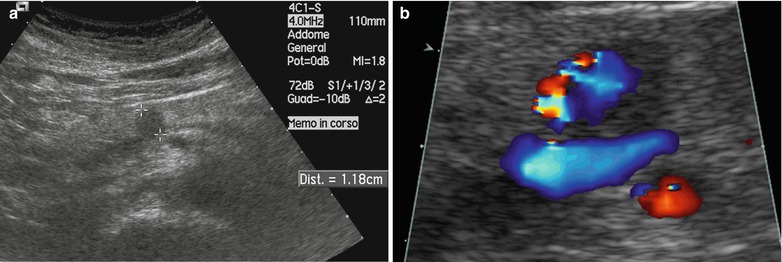
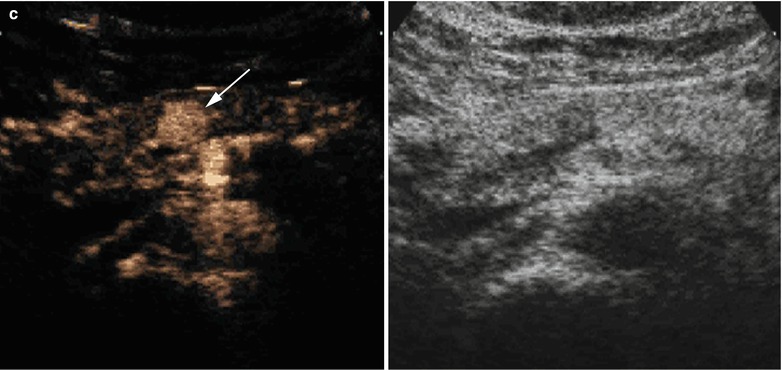
Fig. 3
Insulinoma. (a) Conventional ultrasound: a small hypoechoic round nodule (calipers) with sharp margins within the pancreatic body. (b) Color Doppler ultrasound: positive examination with arterial vessels within the nodule. (c) CEUS: nodule marked enhancement, resulting hyperechoic (arrow) in the arterial phase
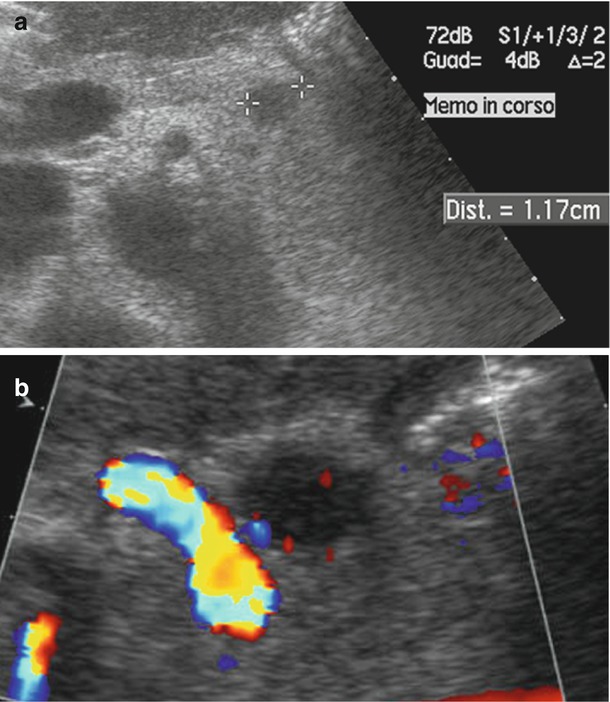
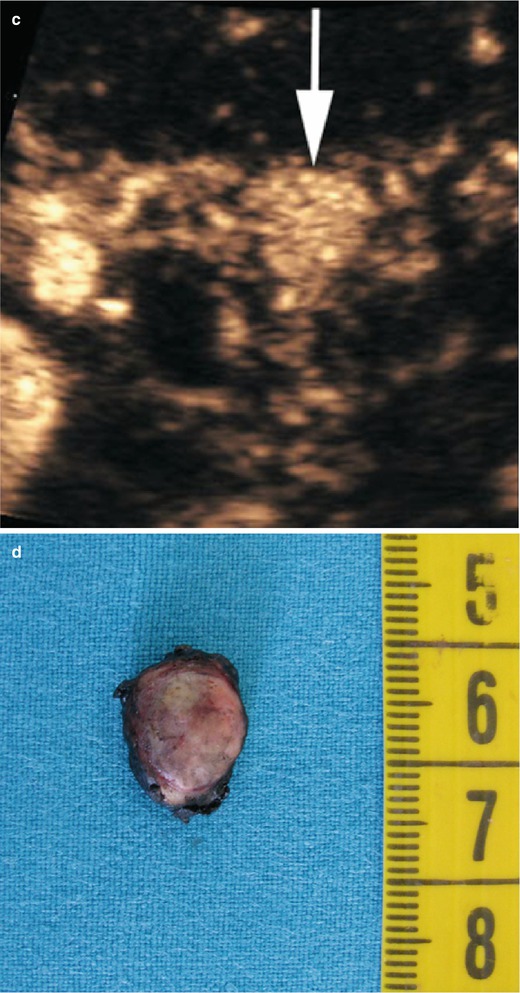
Fig. 4
Insulinoma. (a) Conventional ultrasound: small hypoechoic round nodule (calipers) with sharp margins in the pancreatic body. (b) Color Doppler ultrasound: slightly positive examination with few vessels within the nodule. (c) CEUS: nodule marked enhancement, resulting hyperechoic (arrow) in the arterial phase. (d) Surgical specimen (enucleation): small nodule of 1 cm
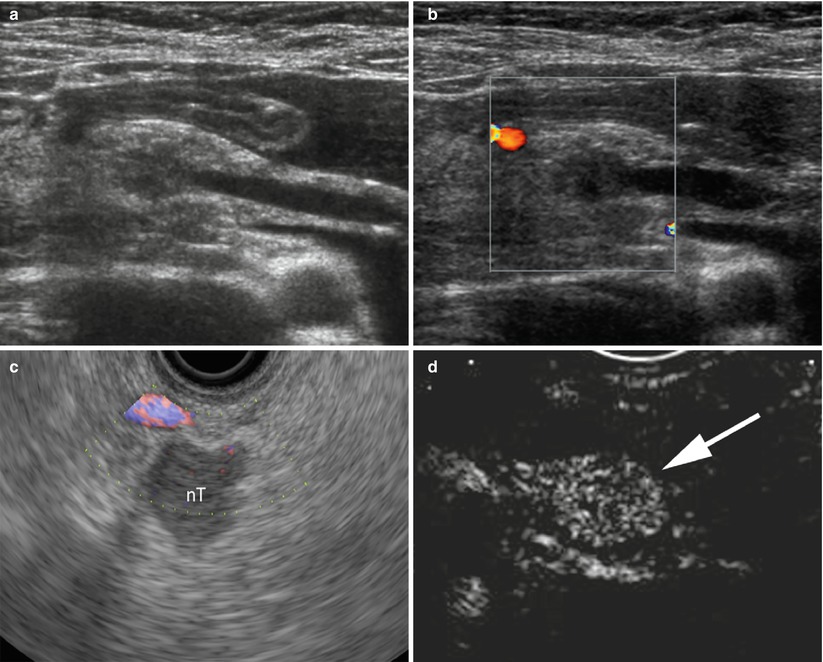
Fig. 5
Small neuroendocrine neoplasms. (a) High-frequency conventional ultrasound: small hypoechoic round nodule in the pancreatic head with upstream dilation of the main pancreatic duct. (b) Color Doppler ultrasound: negative examination with no vessels within the nodule. (c) High-frequency endoscopic color Doppler ultrasound of different case: mainly negative examination, with no significant vessels showed within a hypoechoic nodule (nT in c) with sharp margins. (d) At contrast-enhanced endoscopic ultrasonography, the nodule shows high vascularity becoming hyperechoic (arrow in d) in the arterial phase
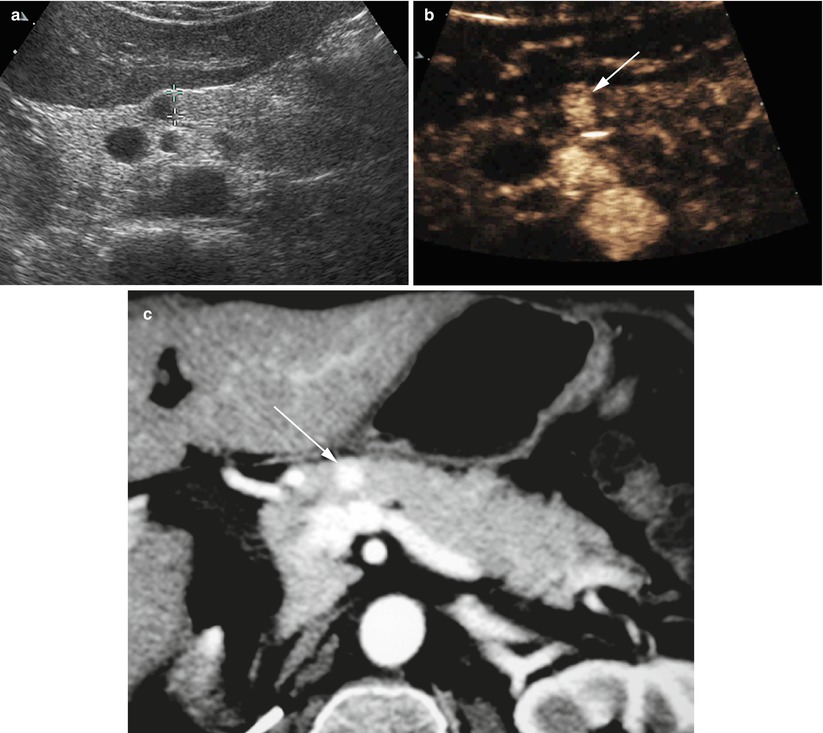
Fig. 6
Insulinoma. (a) Conventional ultrasound: a small hypoechoic round nodule (calipers) with sharp margins in the pancreatic neck. (b, c) CEUS and CT study: the nodule is markedly hypervascular at CEUS (arrow in b) and markedly hypervascular (arrow in c) at CT
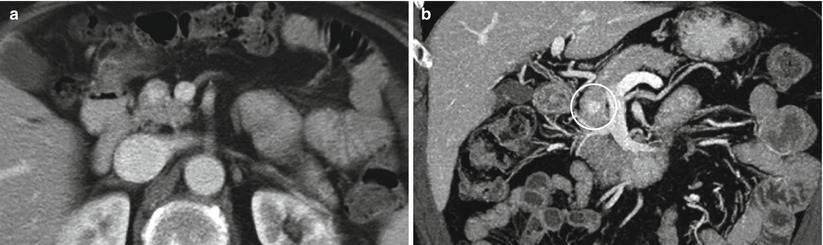
Fig. 7
Insulinoma. (a) CT study: a small hypervascular round nodule in the pancreatic head. (b) CT multiplanar reconstructions: the view on the coronal plane with maximum intensity projection (MIP) algorithm makes the lesion (circle) better detectable
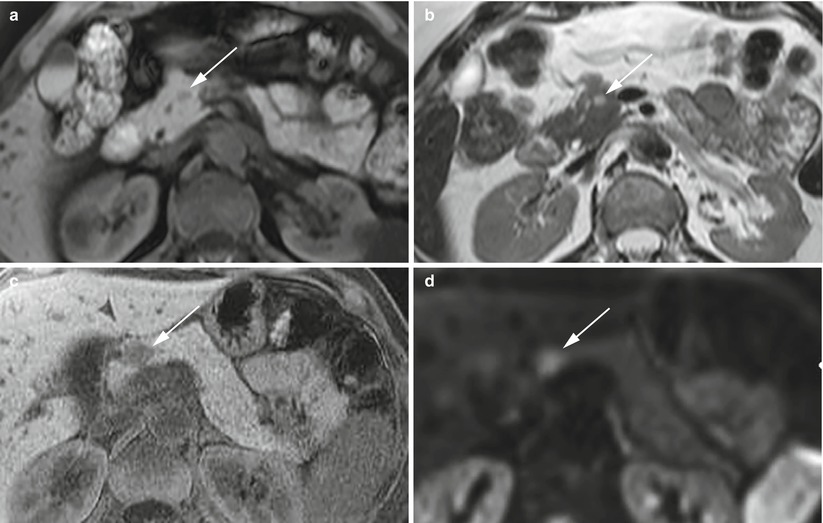
Fig. 8
Insulinoma: typical MRI features. (a, b) Typical MRI aspect: small insulinoma of the pancreatic head, appearing hypointense (arrow in a) in respect to the surrounding pancreatic parenchyma on T1-weighted fat-saturated axial images and hyperintense (arrow in b) on T2-weighted axial images. (c, d) Typical MRI aspect of a different case: small insulinoma of the pancreatic neck, hypointense (arrow in c) on T1-weighted fat-saturated axial images and with diffusion restriction, resulting hyperintense (arrow in d) on DWI with high b values
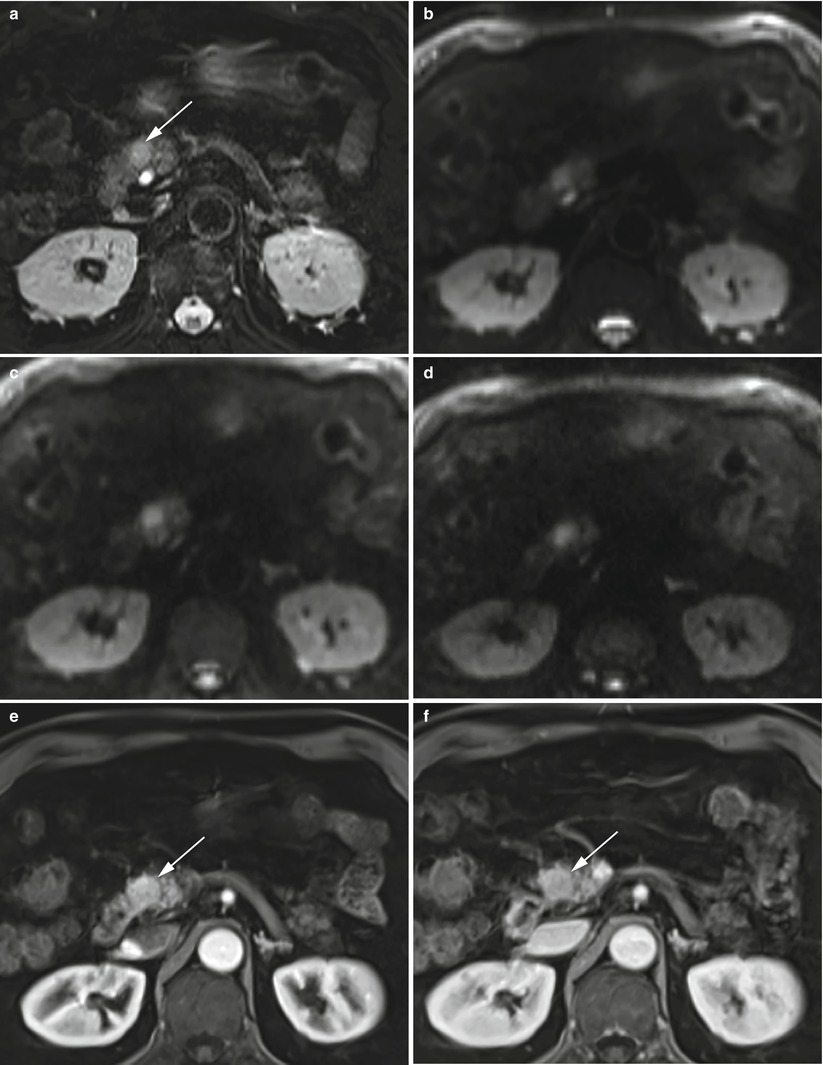
Fig. 9
Insulinoma: typical MRI features. (a–d) Typical MRI aspect: small insulinoma of the pancreatic head, hyperintense (arrow in a) on T2-weighted fat-saturated axial images with typical diffusion restriction (b–d) on DWI (b = 50; b = 400; b = 800). (e, f) Dynamic MRI: study demonstrates hypervascularization of the nodule (arrow in e and f)
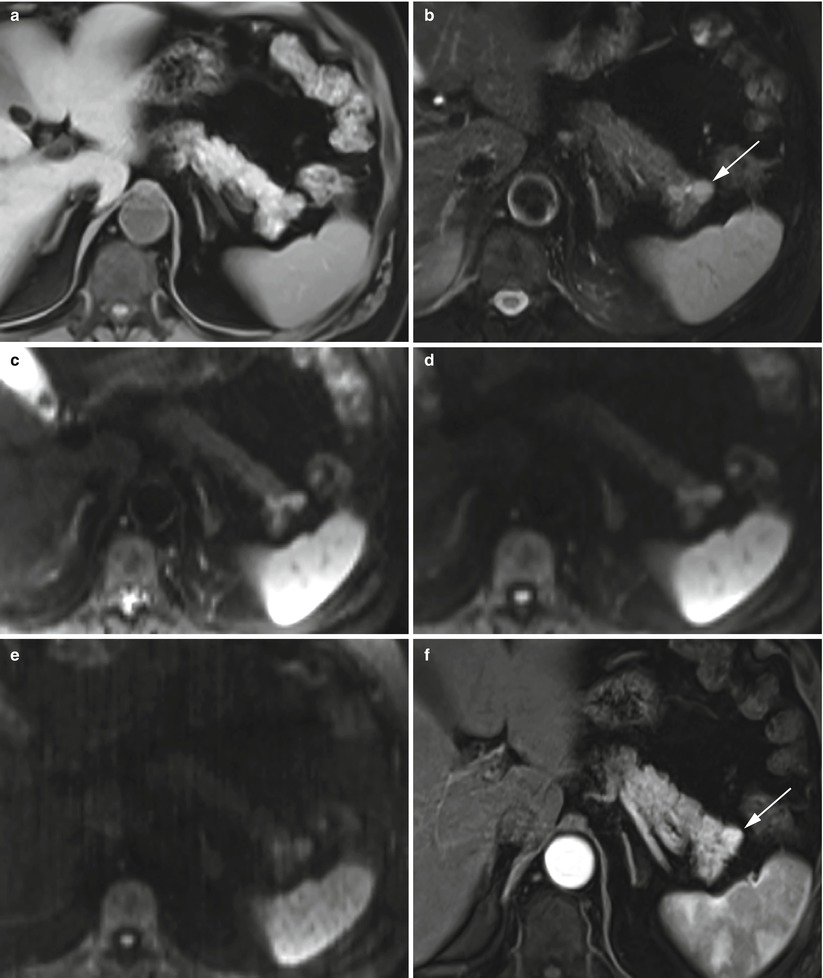
Fig. 10
Insulinoma: typical MRI features. (a, b) Typical MRI aspect: small insulinoma of the pancreatic tail, slightly hypointense on T1-weighted fat-saturated axial images (a) and hyperintense (arrow in b) on T2-weighted fat-saturated axial images (b). (c–e) DWI study: the lesion presents diffusion restriction on progressive increasing b values (b = 50; b = 400; b = 800). (f) Dynamic MRI: study shows hypervascularity of the nodule (arrow in f) in respect to the surrounding pancreatic parenchyma
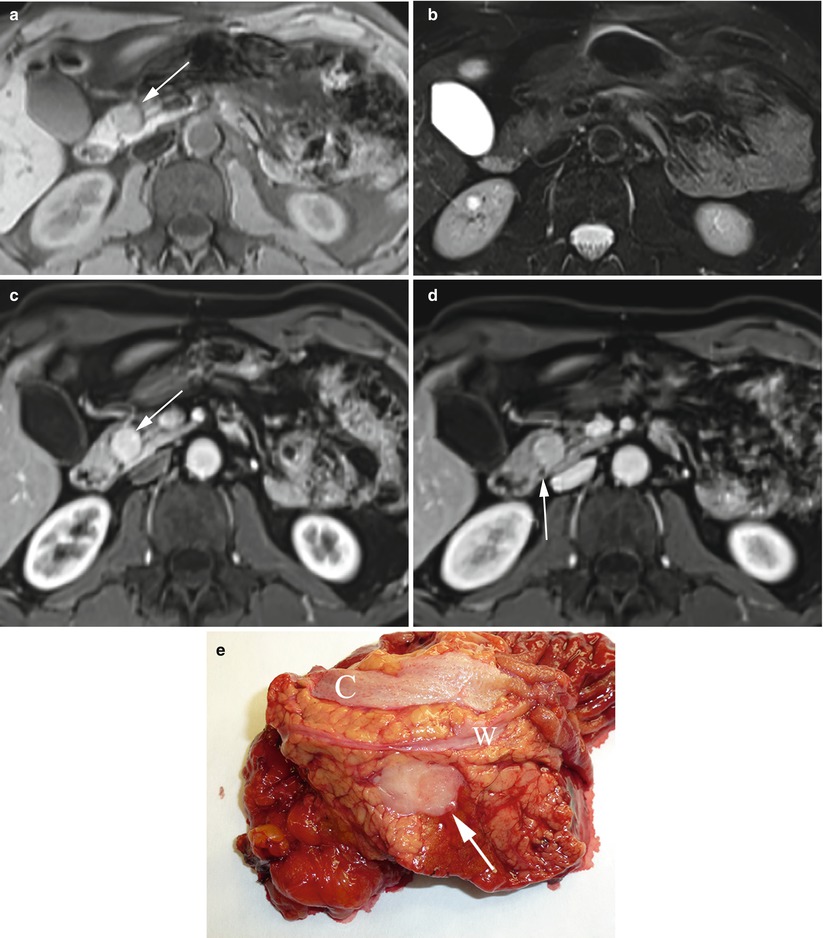
Fig. 11
Small neuroendocrine neoplasm: atypical MRI features and relationship with the main pancreatic duct. (a, b) Atypical MRI aspect: round-shaped insulinoma of the pancreatic head, slightly hypointense (arrow in a) on T1-weighted fat-saturated axial images (a) and isointense on T2-weighted fat-saturated axial images (b). (c, d) Dynamic MRI: study demonstrates typical hypervascularity of the nodule (arrow in c) in respect to the surrounding pancreatic parenchyma. The lesion appears to be strictly close to the main pancreatic duct (arrow in d) making enucleation impossible. (e) Surgical specimen (pancreaticoduodenectomy): small round nodule (arrow) strictly close to the main pancreatic duct (W). Common bile duct (C) is visible
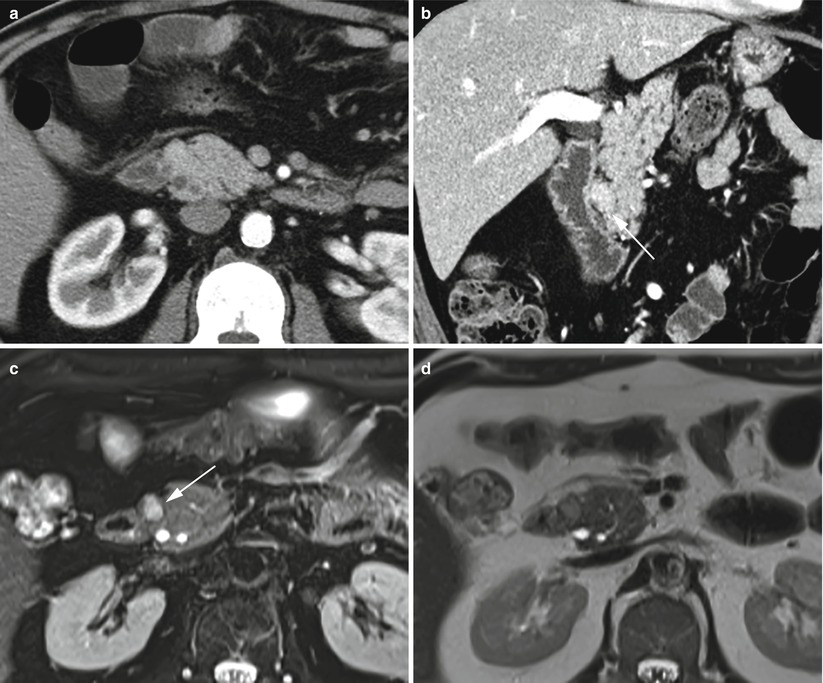
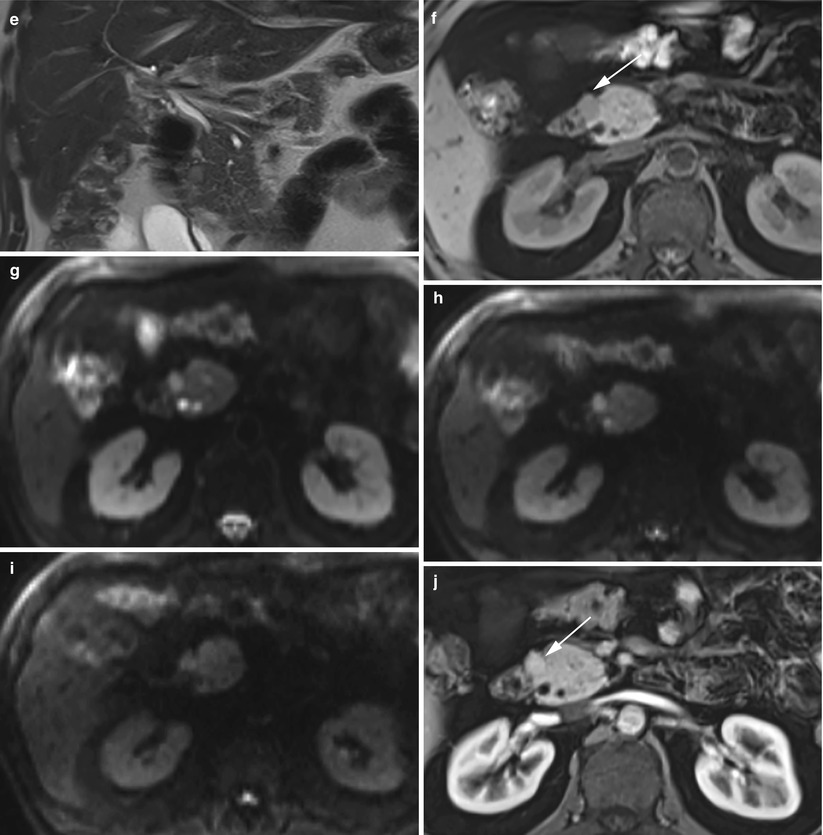
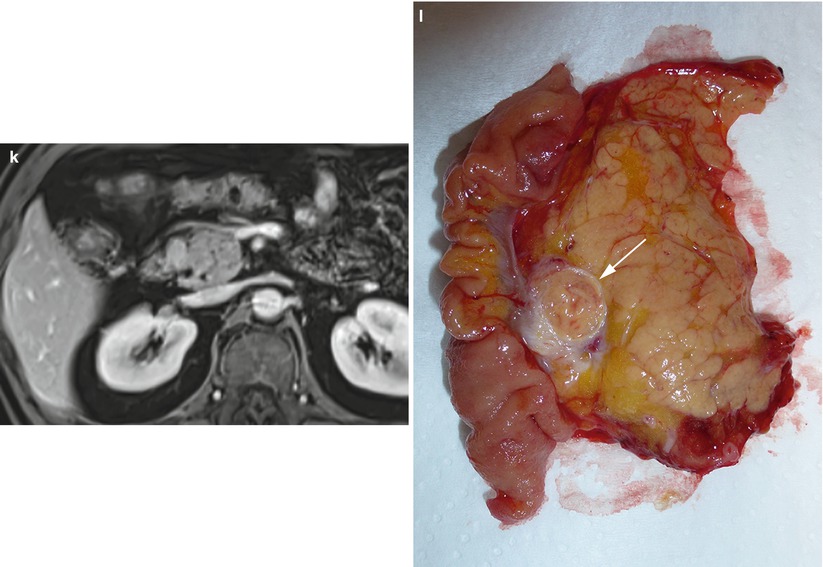
Fig. 12
Groove small neuroendocrine neoplasm. (a, b) Dynamic CT: a small round-shaped slightly hypervascular nodule is better visible on the multiplanar reconstruction coronal plane (arrow in b), exactly in the paraduodenal space. (c–k) Complete MRI study: the nodule is markedly hyperintense (arrow in c) on T2-weighted fat-saturated axial images and slightly hyperintense on T2-weighted axial (d) and coronal (e) images without fat saturation. Slight hypointensity of the lesion (arrow in f) on T1-weighted fat-saturated axial images. The lesion presents diffusion restriction (g–i) on DWI (b = 50; b = 400; b = 800). (j, k) Dynamic MRI demonstrates the hypervascularity of the nodule (arrow in j) in respect to the surrounding pancreatic parenchyma. (l) Surgical specimen (pancreaticoduodenectomy): the presence of a small capsulated nodule (arrow) is visible in close relationship with the duodenal wall within the groove
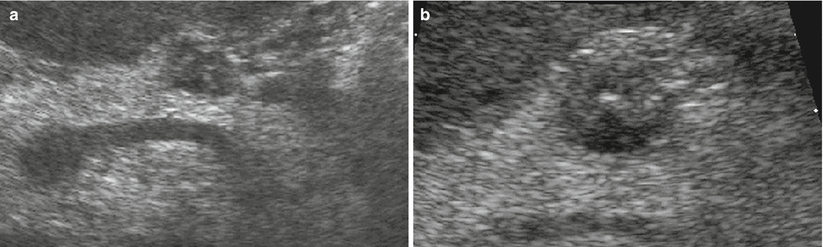
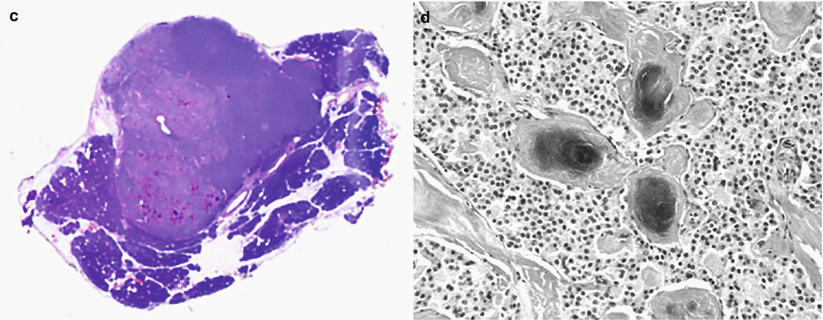
Fig. 13
Insulinoma with small calcifications. (a, b) Conventional ultrasound: examination shows a small hypoechoic lesion bulging from the ventral surface of the pancreatic body. In the zoomed image (b), small calcifications within the lesion, appearing as small hyperechoic spots, are visible. (c, d) Macrosection and hystopathology of the surgical specimen: typical lesion with high cellularity and with small calcifications
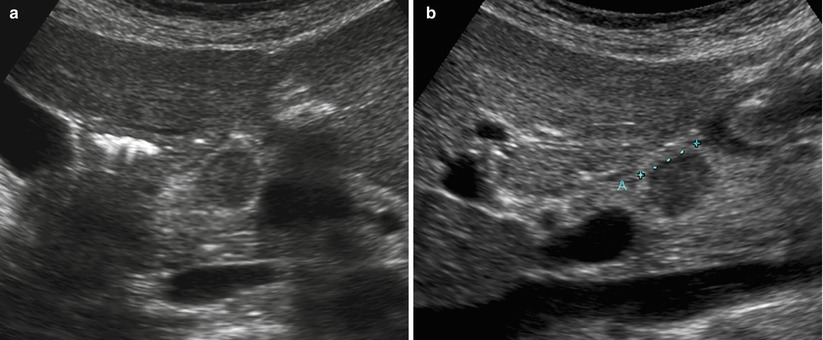
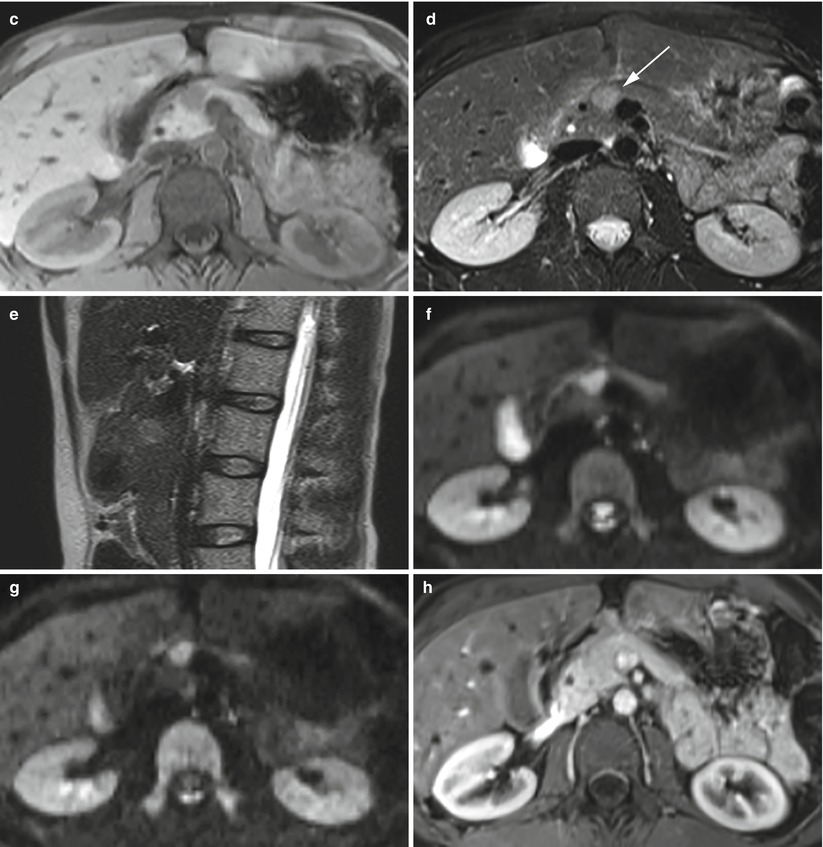
Fig. 14
Isovascularized small neuroendocrine neoplasm. (a, b) Conventional ultrasound: examination (a, axial and b, sagittal planes) shows a hypoechoic round-shaped nodule (calipers in b) with sharp margins in the pancreatic neck. (c–h) MRI examination: the lesion appears hypointense on T1-weighted fat-saturated axial images (c) and hyperintense (arrow in d) on T2-weighted fat-saturated and not saturated sagittal images (e). The nodule typically presents diffusion coefficient restriction (f, g) on DWI (b = 400; b = 800). At dynamic MRI (h), the lesion is hardly recognizable, being isovascularized in respect to the surrounding pancreatic parenchyma
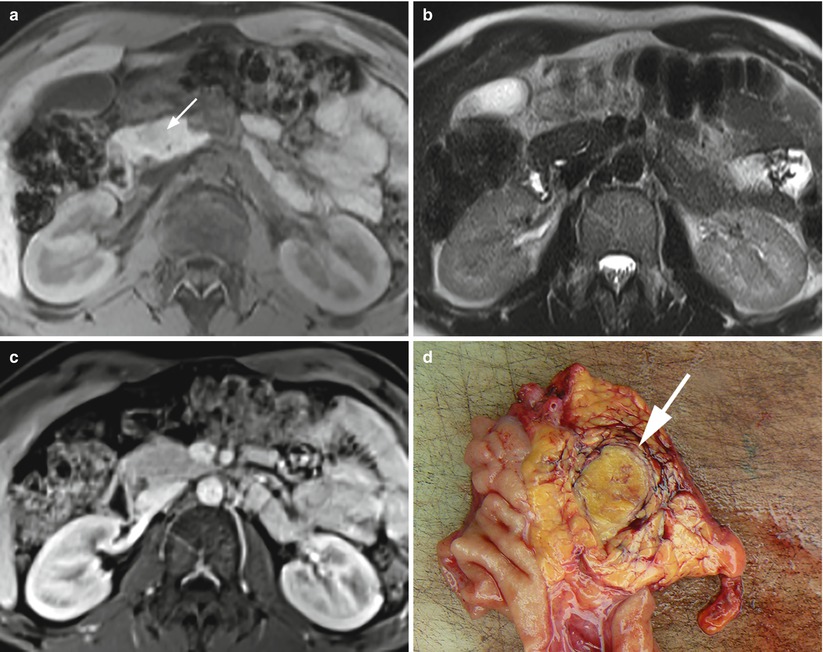
Fig. 15
Hypovascularized insulinoma. (a–c) MRI study: round-shaped nodule of the pancreatic head, slightly hypointense (arrow in a) on T1-weighted axial images and almost isointense on T2-weighted fat-saturated axial images (b). At dynamic MRI study (c), the lesion atypically appears isointense in respect to the surrounding pancreatic parenchyma. (d) Surgical specimen (pancreaticoduodenectomy): small capsulated insulinoma (arrow) of the pancreatic head
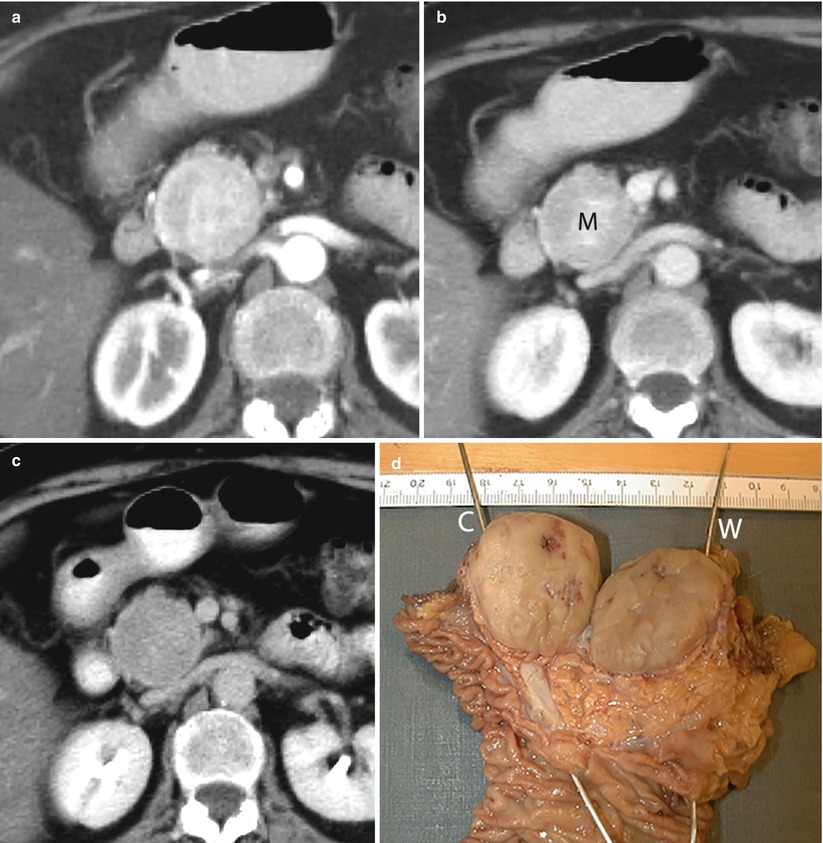
Fig. 16
VIPoma. (a–c) CT dynamic study: large round-shaped capsulated mass (M in b) with sharp margins in the pancreatic head. Surrounding anatomical structures are displaced rather than infiltrated by the lesion. A slightly inhomogeneous hypervascularization of the mass with a progressively increasing conspicuity of the capsule is demonstrated. (d) Surgical specimen (pancreaticoduodenectomy): large well circumscribed pancreatic mass with final diagnosis of VIPoma. The probe in the common bile duct (C) ends in the papilla major. The probe in the Wirsung duct (W) ends in the papilla minor
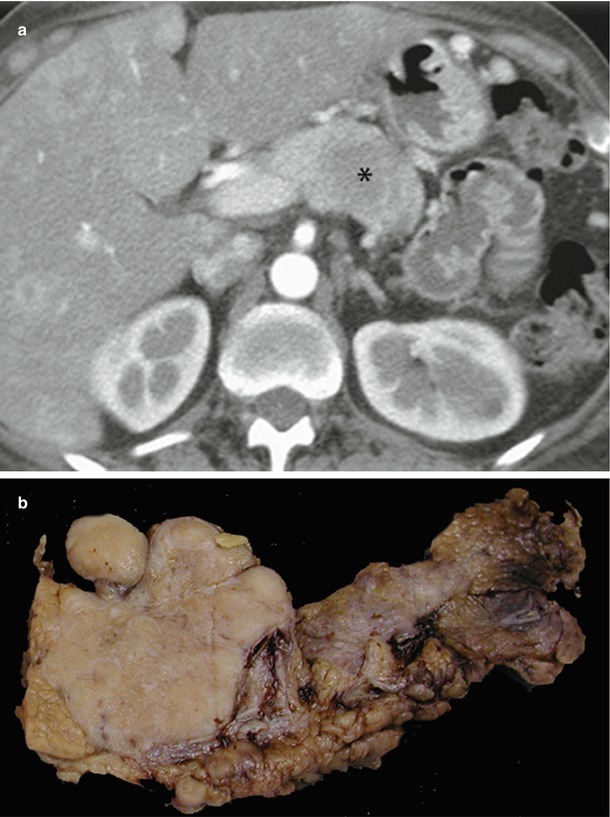
Fig. 17
ACTHoma. (a) Dynamic CT: the study shows a large round-shaped lesion with irregular margins in the pancreatic body, resulting hypovascular (asterisk) in respect to surrounding pancreatic parenchyma in the postcontrastographic arterial phase. Also, metastatic liver lesions are detectable. (b) Surgical specimen (distal pancreatectomy): large firm pancreatic mass with final diagnosis of ACTH-producing neuroendocrine tumor
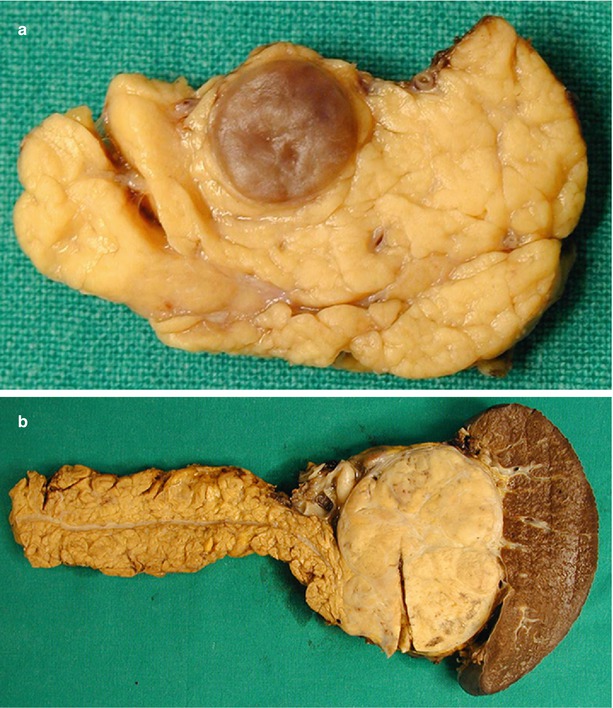
Fig. 18
Nonfunctioning neuroendocrine neoplasm. (a) Surgical specimen (intermediate pancreatectomy): typical macroscopic aspect of a small nonfunctioning neuroendocrine neoplasm, presenting as a small well-demarcated nodule with regular margins. (b) Surgical specimen (distal pancreatectomy): typical macroscopic aspect of a large nonfunctioning neuroendocrine neoplasm, presenting as a large well-demarcated mass with regular margins
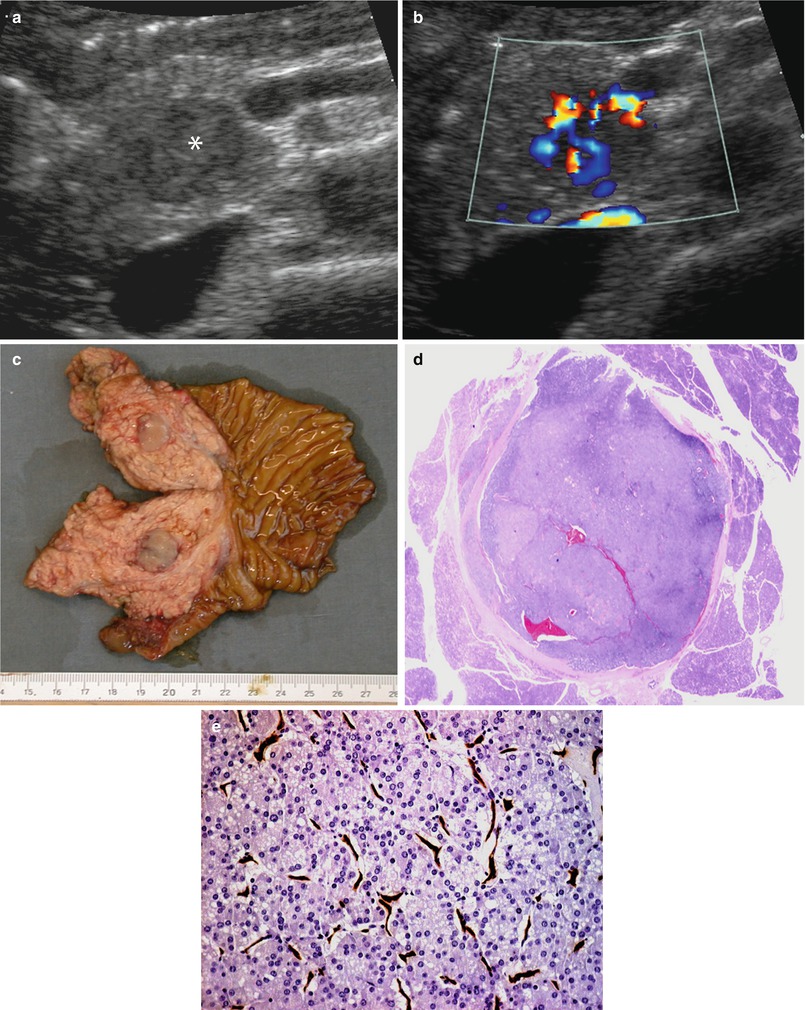
Fig. 19
Nonfunctioning small neuroendocrine neoplasm. (a, b) Conventional and color Doppler US examinations: small hypoechoic round-shaped nodule (asterisk in a) with sharp margins in the pancreatic head. Intense positivity at color Doppler (b) analysis is visible. (c) Surgical specimen (pancreaticoduodenectomy): small well-defined round-shaped lesion in the pancreatic head. (d, e) Histopathology: small round-shaped lesion (d) with diagnosis of neuroendocrine neoplasm (e), showing high cellularity at hematoxylin and eosin staining and high intralesional vascular network, demonstrated by CD34 immunohistochemical staining (brown traces in e)
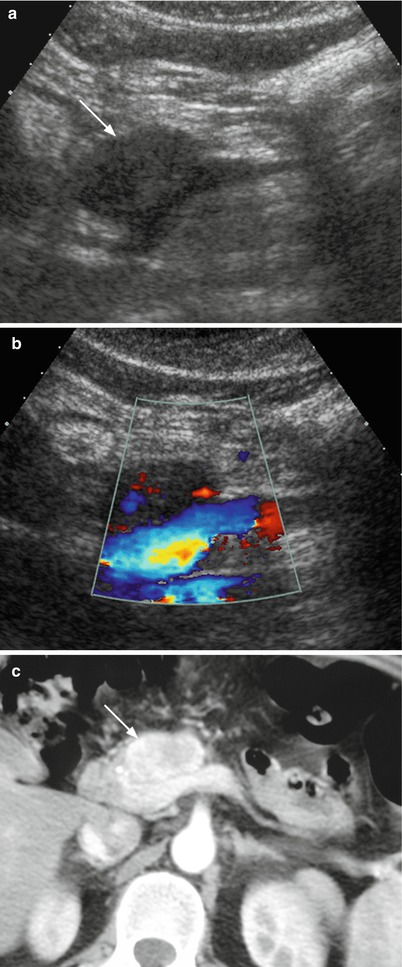
Fig. 20
Nonfunctioning neuroendocrine tumor. (a, b) Conventional and color Doppler US examinations: round-shaped hypoechoic lesion (arrow in a) with sharp margins in the pancreatic neck involving the spleno-portal venous confluence. Color Doppler analysis (b) shows only few vessels within the lesion. (c) Dynamic CT: the lesion typically appears inhomogeneously hyperenhancing (arrow) in respect to surrounding pancreatic parenchyma
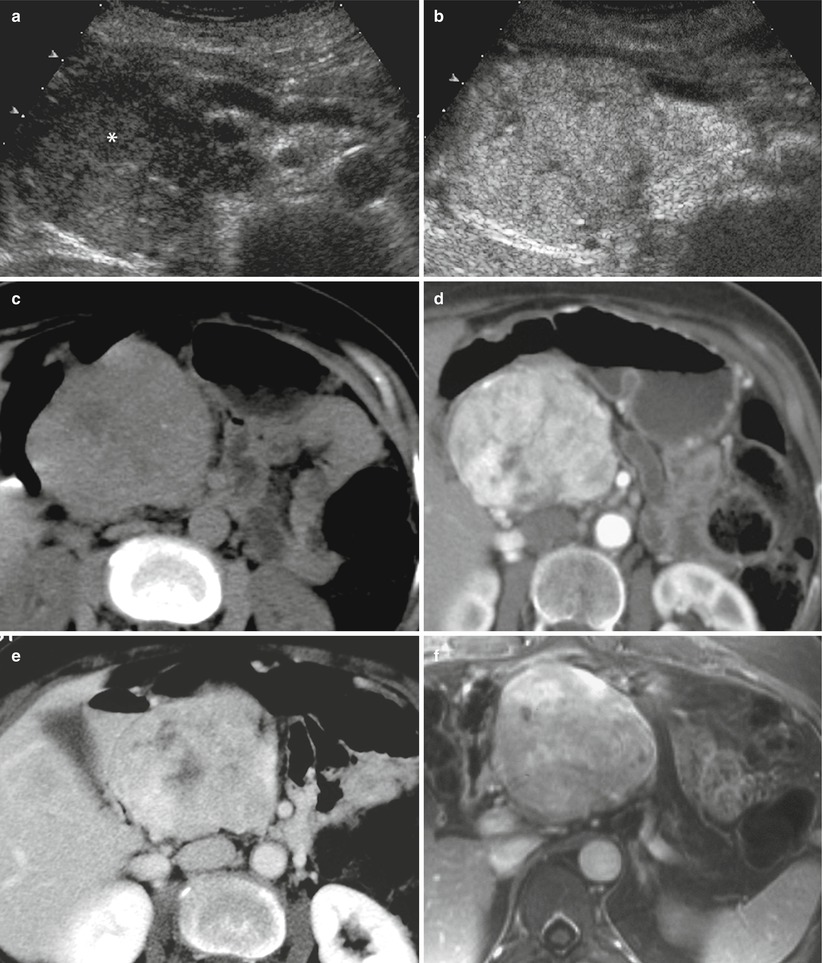
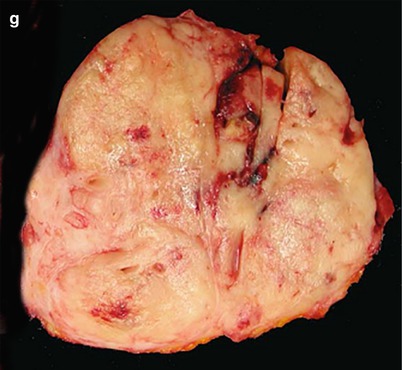
Fig. 21
Nonfunctioning neuroendocrine tumor. (a, b) US and CEUS examinations: large hypoechoic mass (asterisk in a) with small calcifications in the pancreatic head, causing upstream dilation of the Wirsung duct. This lesion is inhomogeneously hypervascularized at CEUS (b). (c–e) CT examination: the pancreatic mass appears hypodense with small calcifications in the precontrastographic phase (c) and inhomogeneously hyperenhancing in respect to the surrounding pancreatic parenchyma in the dynamic phases (d–e). (f) Dynamic MRI: inhomogeneous hypervascularity of the lesion. (g) Surgical specimen (distal pancreatectomy) of different case: large well clear-cut margins firm mass with final diagnosis of neuroendocrine tumor
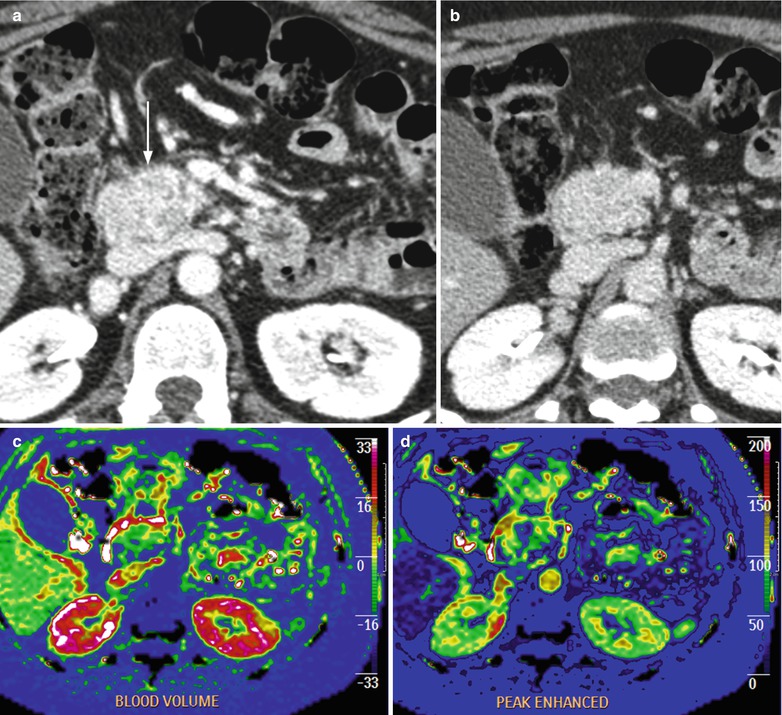
Fig. 22
Nonfunctioning neuroendocrine tumor. (a, b) Dynamic CT: oval-shaped lesion with slightly irregular margins in the pancreatic head, endorsed on the ventral surface of inferior vena cava, hyperenhancing (arrow in a) in the postcontrastographic phases. (c, d) Perfusion CT: color-map analysis demonstrates high blood volume and peak enhancement values of the lesion
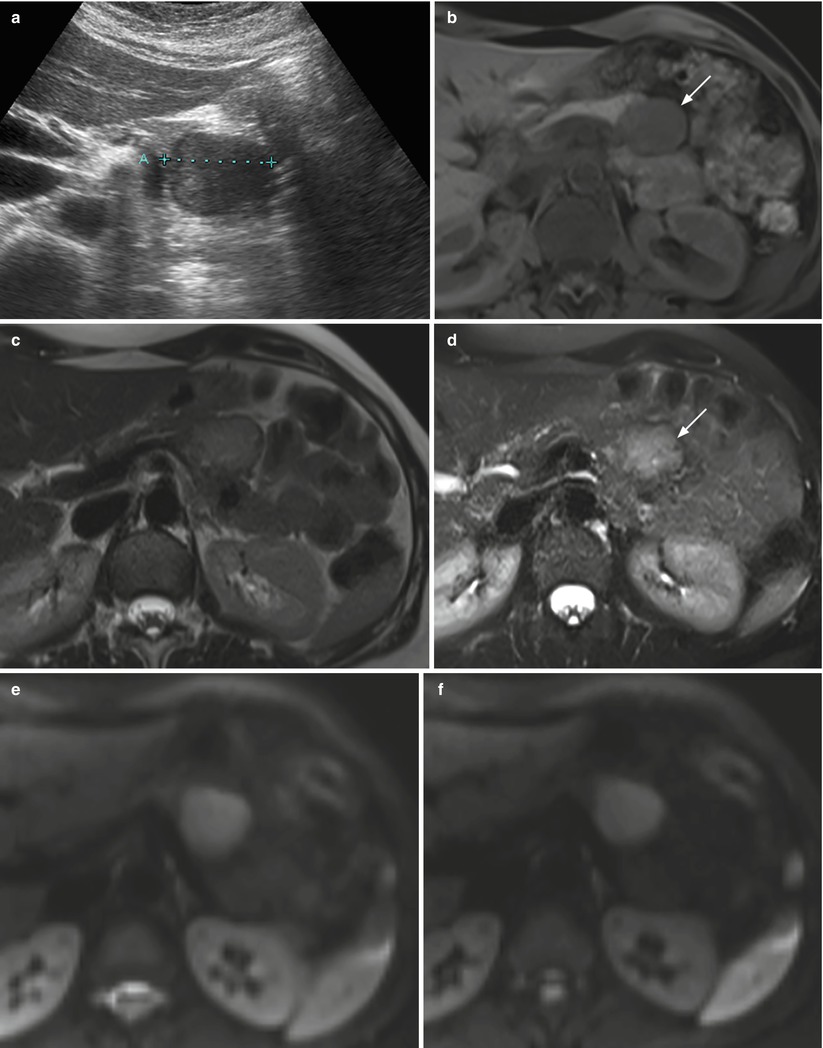
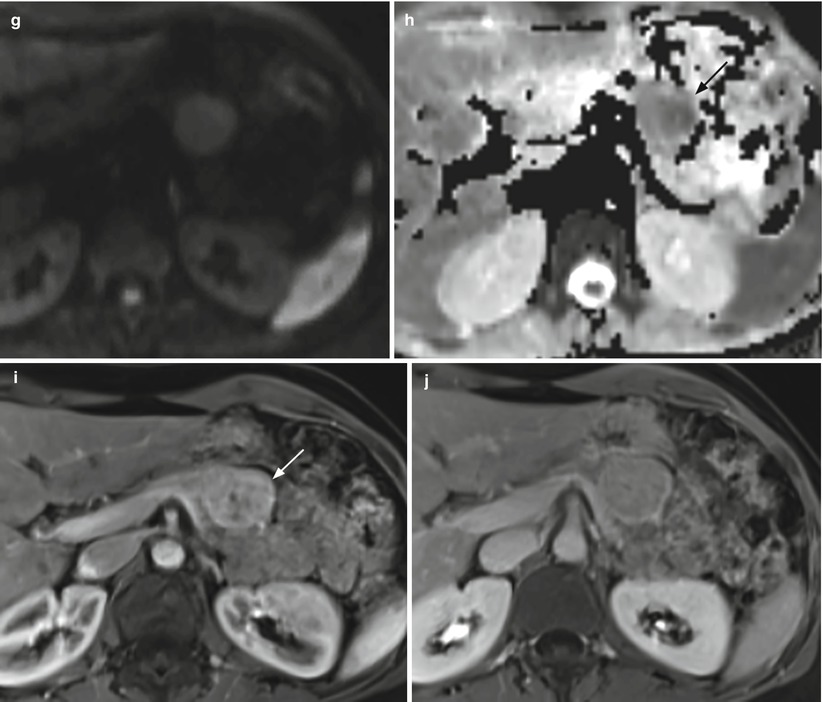
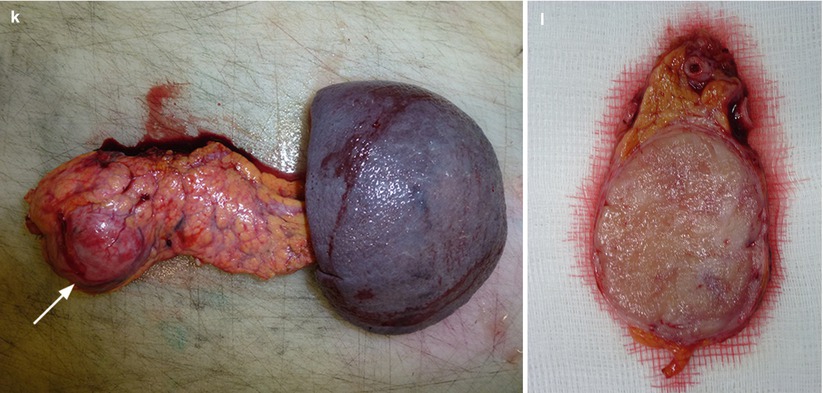
Fig. 23
Nonfunctioning neuroendocrine tumor. (a) Conventional US examination: hypoechoic round-shaped capsulated lesion (calipers) with sharp margins bulging from the surface of the pancreatic body. (b–d) MRI examination: the lesion presents hypointensity (arrow in b) in respect to the surrounding pancreatic parenchyma on T1-weighted fat-saturated images and hyperintensity on T2-weighted images, both without (c) and with fat-saturation (arrow in d). (e–h) DWI study: the lesion also shows diffusion restriction (b = 50; b = 400; b = 800) and hypointensity (arrow in h) on ADC map. (i, j) Dynamic MRI: the lesion is hypervascular (arrow in i) in respect to the surrounding pancreatic parenchyma, with increasing conspicuity of the capsule on the venous phase (j). (k, l) Surgical specimen (distal pancreatectomy): the lesion is clearly visible (arrow in k), bulging from the anterior surface of the pancreatic body and appearing well demarcated on the cut section (l)
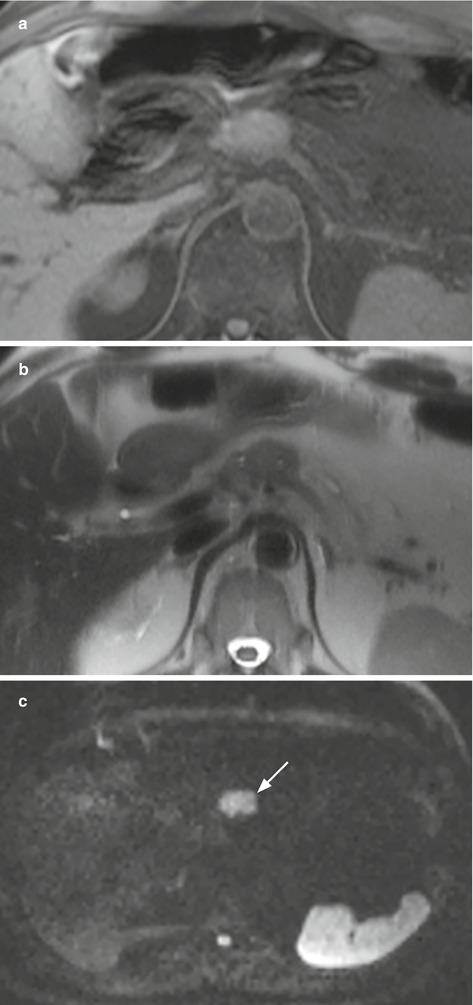
Fig. 24
Nonfunctioning neuroendocrine neoplasm at DWI. (a, b) MRI examination: pancreatic neck isointense lesion both on T1-weighted fat-saturated and T2-weighted images. The lesion is clearly visible, showing typical diffusion restriction, appearing hyperintense (arrow in c) on DWI
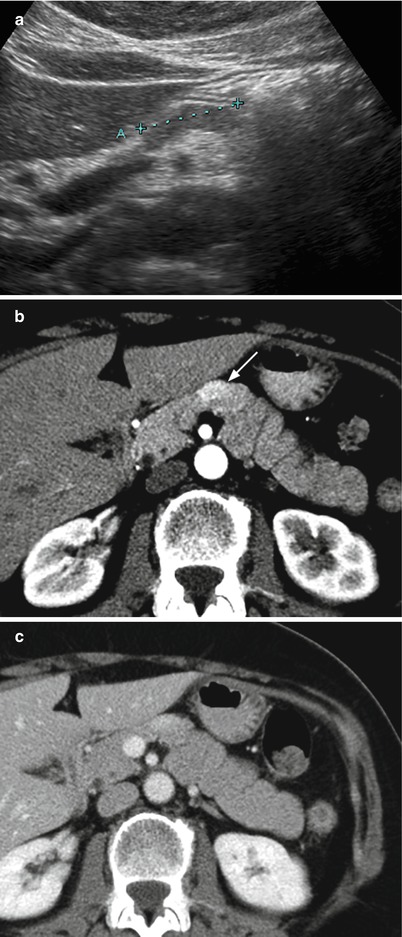
Fig. 25
Nonfunctioning neuroendocrine tumor with atypical shape. (a) Conventional US examination: hypoechoic elongated lesion (calipers) with sharp margins on the ventral surface of the pancreatic neck. (b, c) Dynamic CT: the lesion typically appears hypervascular (arrow in b)
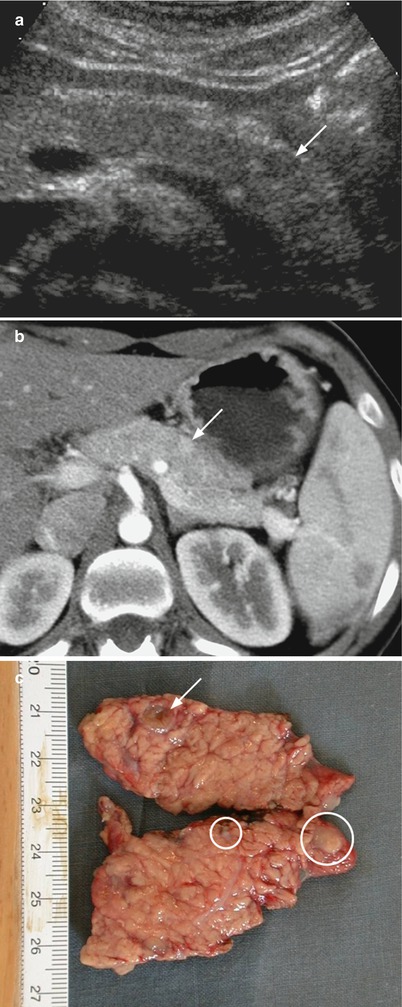
Fig. 26
Multiple small neuroendocrine neoplasms. (a) Conventional US examination: small hypoechoic nodule with sharp margins (arrow) on the ventral surface of the pancreatic body is visible. (b) Dynamic CT: the small lesion is hypervascular (arrow) in respect to the surrounding pancreatic parenchyma. No other lesions are clearly visible. (c) Surgical specimen (distal pancreatectomy): the lesion (arrow) is confirmed at the pancreatic body, but other smaller lesions (circles), not recognizable at preoperative imaging, can be seen
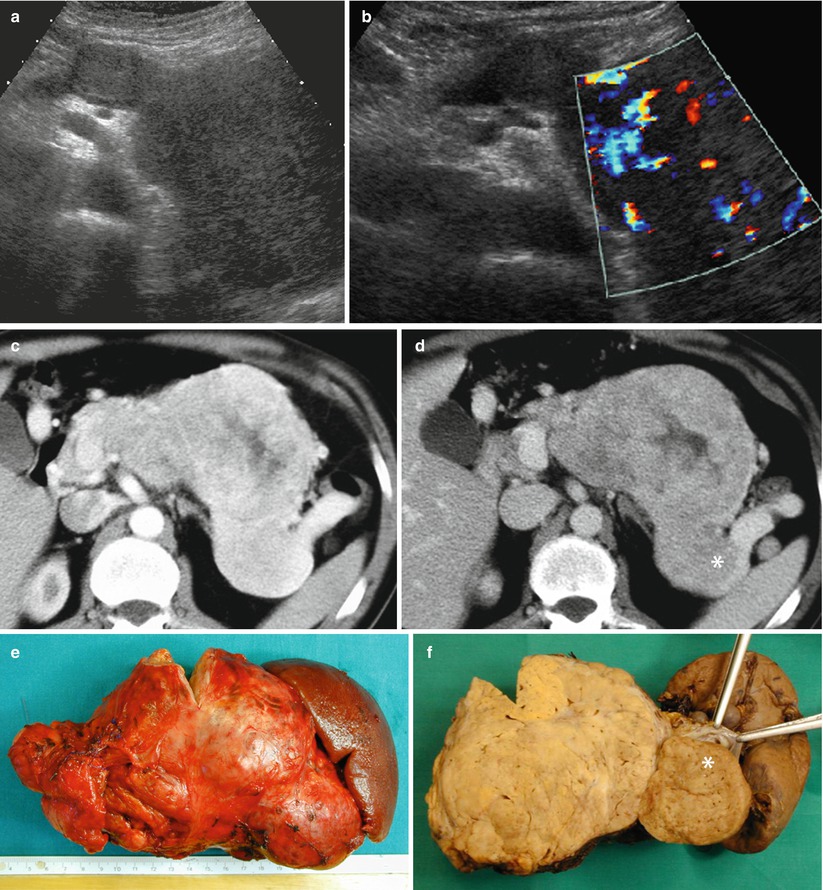
Fig. 27
Giant nonfunctioning neuroendocrine tumor. (a, b) Conventional US examination: huge hypoechoic mass from the neck to the tail of the pancreas with intense positivity at color Doppler analysis (b). (c, d) Dynamic CT: the huge lesion appears inhomogeneously hypervascular and with intravascular growth colonizing the splenic vein (asterisk in d). (e, f) Surgical specimen (distal pancreatectomy): huge mass (e) with voluminous neoplastic thrombus (asterisk in f) into the splenic vein
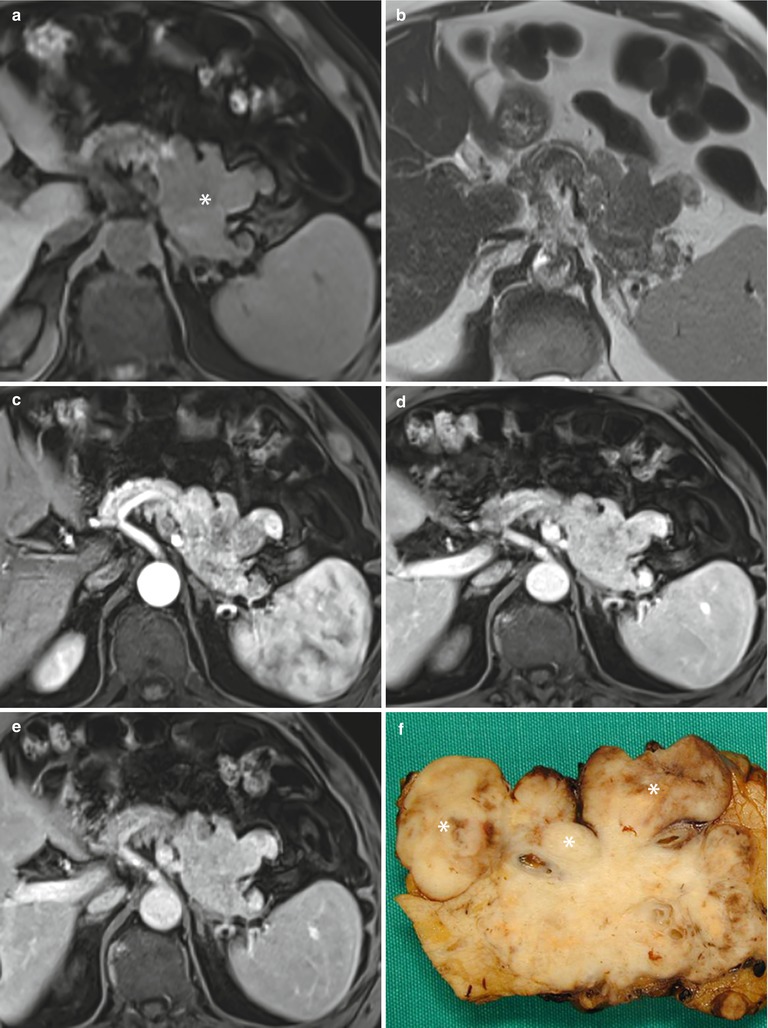
Fig. 28
Nonfunctioning neuroendocrine tumor with irregular margins. (a, b) MRI study: large irregular-shaped lesion (asterisk in a) of the body–tail of the pancreas, with protruding finger-like portion, growing in the mesenteric fat tissue and in the retroperitoneal space, hypointense in respect to the surrounding pancreatic parenchyma on T1-weighted fat-saturated axial images (a) and isointense on T2-weighted axial images (b). (c–e) Dynamic MRI: this irregular mass appears inhomogeneously hypervascular (c) with a progressive enhancement homogenization in the venous (d) and late (e) phases. (f) Surgical specimen (distal pancreatectomy) of different case, showing large nonfunctioning irregular-shaped tumor of the pancreas with protruding finger-like neoplastic tissue, resulting from the nodal involvement (asterisks) of the tumor
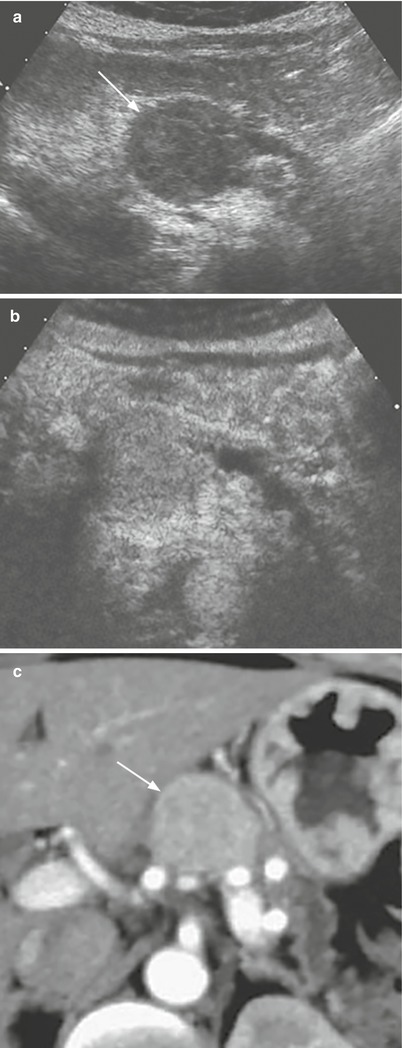
Fig. 29
Nonfunctioning well-differentiated neuroendocrine tumor. (a, b) US examination: hypoechoic round-shaped capsulated lesion (arrow in a) of the pancreatic body, with upstream dilation of the Wirsung duct. At CEUS (b), the lesion appears hypervascularized in respect to the surrounding pancreatic parenchyma. (c) Dynamic CT: the lesion is homogeneously hypervascularized (arrow)
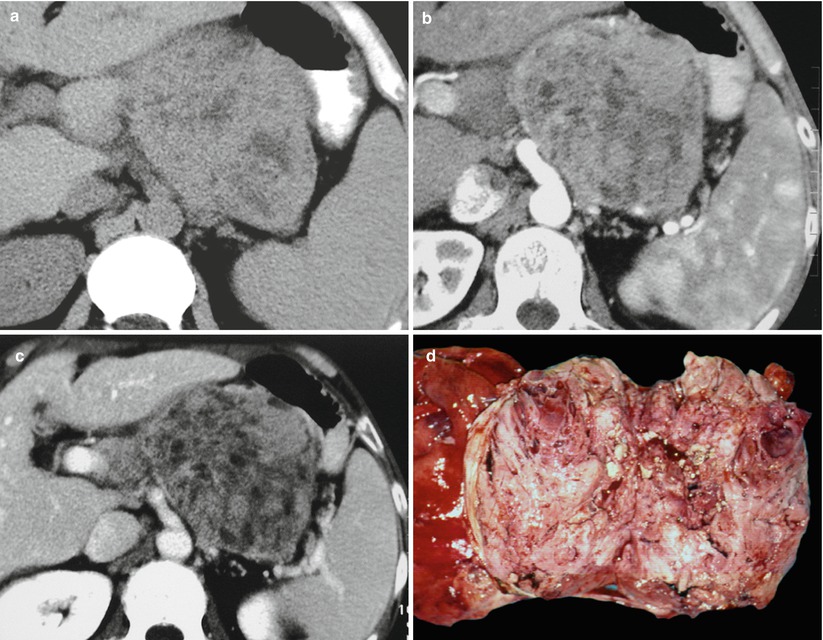
Fig. 30
Nonfunctioning poorly differentiated neuroendocrine tumor. (a–c) CT study: large inhomogeneous mass of the body–tail of the pancreas characterized by markedly inhomogeneous contrast enhancement in the arterial (b) and venous (c) phases. (d) Surgical specimen (distal pancreatectomy): markedly inhomogeneous tumor with necrotic areas
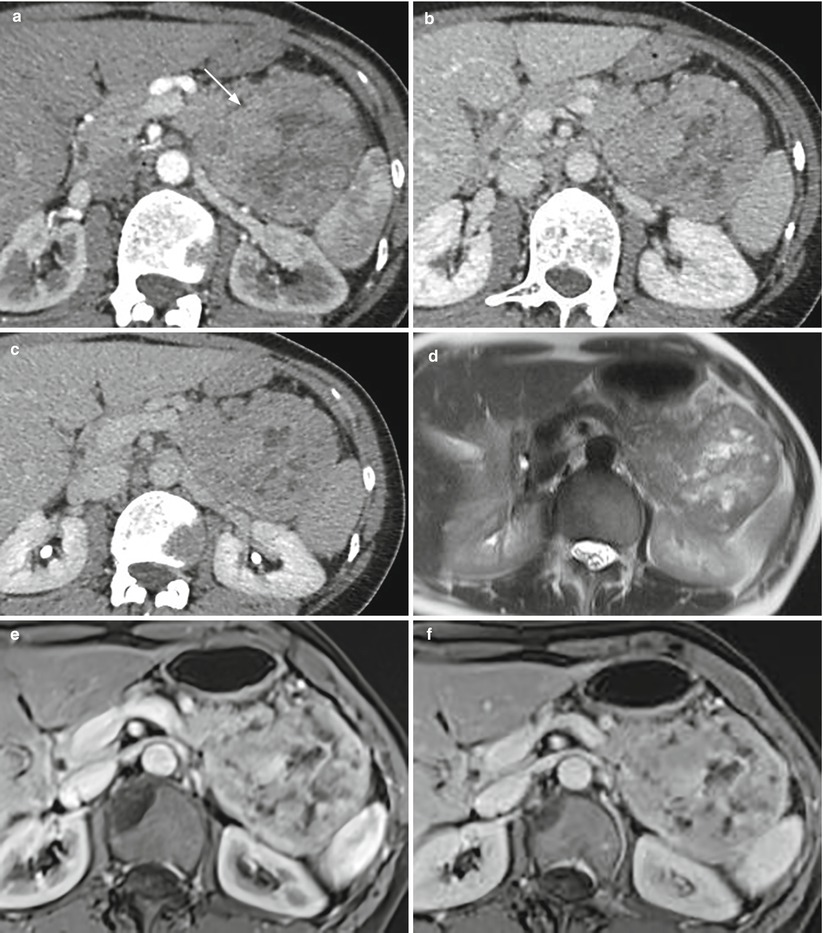
Fig. 31
Neuroendocrine tumor. (a–c) Dynamic CT: large mass (arrow in a) of the body–tail of the pancreas, showing markedly inhomogeneous enhancement in the arterial (a), venous (b), and late (c) phases, due to the presence of multiple intratumoral necrotic areas. (d–f) MRI study: multiple hyperintense areas on T2-weighted images are visible in the mass, resulting inhomogeneous hypervascular in the postcontrastographic arterial (e) and venous (f) phases
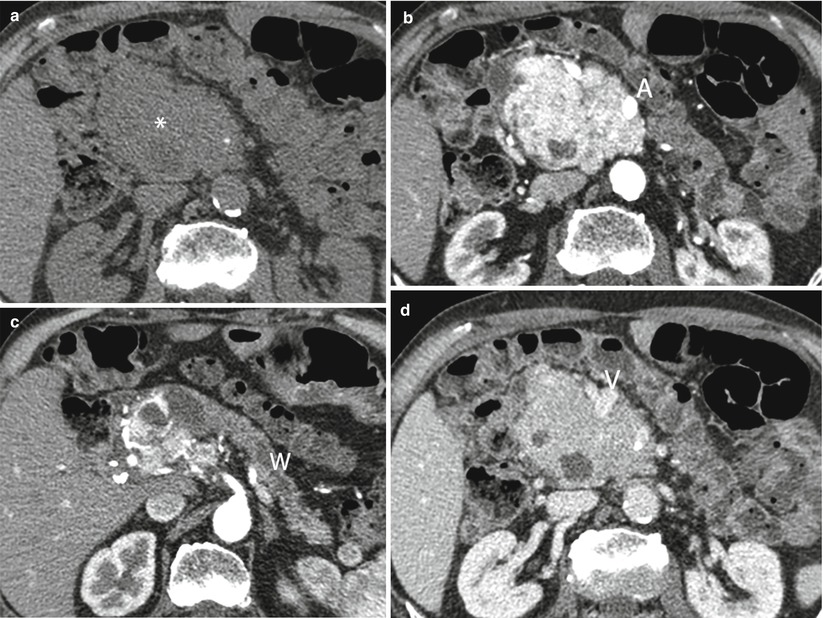
Fig. 32
Locally invasive neuroendocrine tumor. (a–d) Dynamic CT: precontrast-enhanced phase shows a large hypodense mass with irregular margins and calcification in the pancreatic head (asterisk in a), resulting hypervascular in the dynamic phase with infiltration of the superior mesenteric artery (A in b). The main pancreatic duct is upstream dilated (W in c). The mass infiltrates the superior mesenteric vein (V in d)
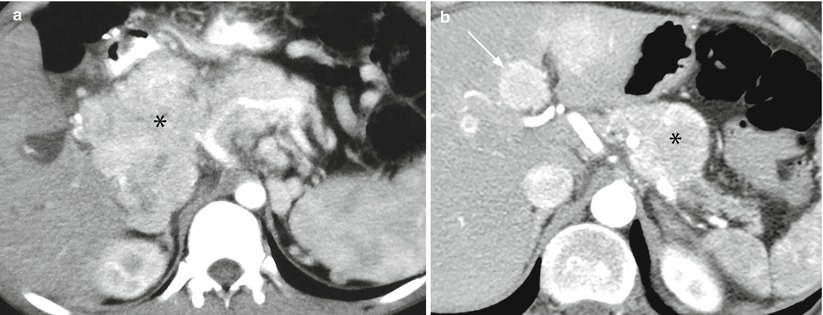
Fig. 33
Locally invasive neuroendocrine tumor and metastatic neuroendocrine tumor. (a) Dynamic CT: large mass (asterisk) with irregular margins, originating from the pancreatic head and body, appearing inhomogeneously hypervascular and infiltrating the celiac trunk and its branches. (b) Dynamic CT: relatively small round-shaped lesion (asterisk) with regular margins of the body of the pancreas, appearing hypervascular and infiltrating the splenic artery. The pancreatic tail parenchyma is atrophic with dilation of the Wirsung duct. Hypervascular liver metastases (arrow) are present
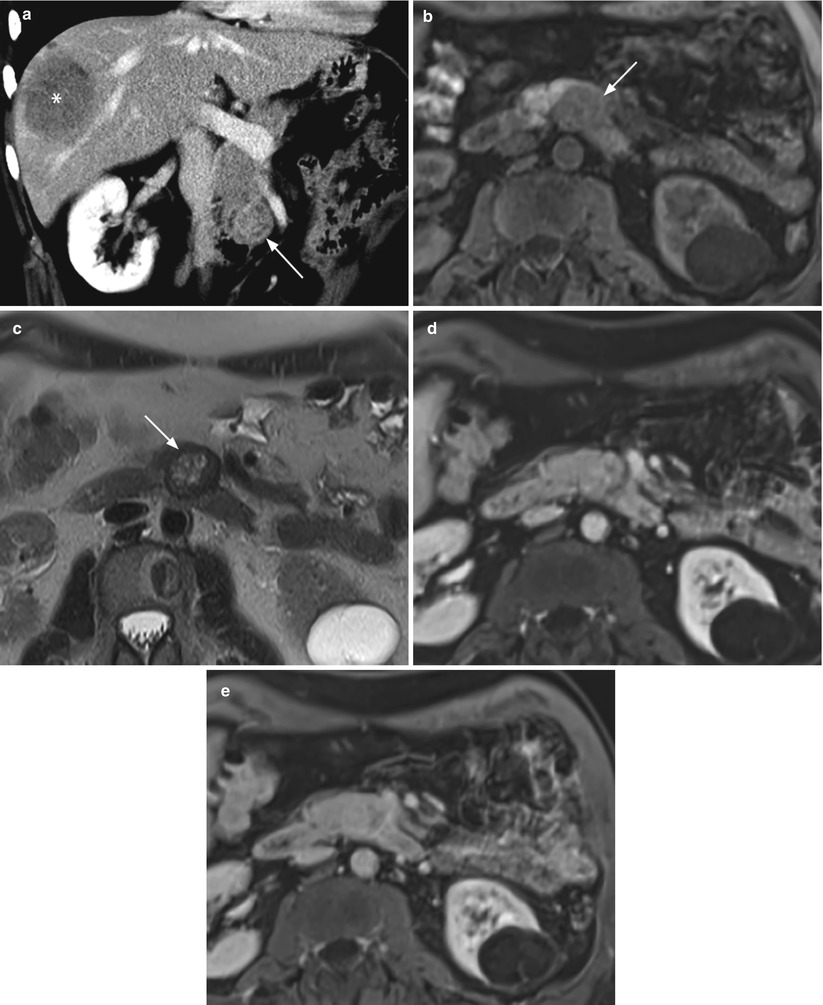
Fig. 34
Small metastatic neuroendocrine tumor. (a) Dynamic CT: small round-shaped lesion (arrow) of the uncinate process of the pancreatic, appearing inhomogeneously but well vascularized. A large hypovascular metastatic lesion (asterisk) is recognizable in the right liver lobe. (b–e) MRI study: the pancreatic tumor is slightly hypointense (arrow in b) on T1-weighted fat-saturated axial images and inhomogeneously hyperintense (arrow in c), with a hypointense peripheral rim, on T2-weighted axial images. At dynamic MRI study, the lesion is almost isovascular in respect to the surrounding pancreatic parenchyma on postcontrastographic arterial (d) and venous (e) phases
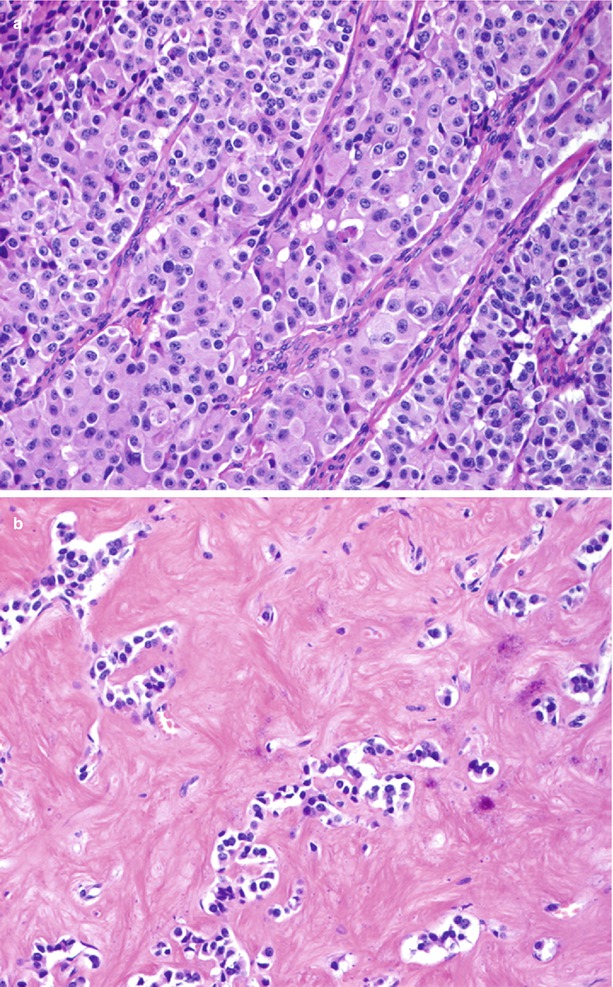
Fig. 35
High-cellularity vs low-cellularity nonfunctioning neuroendocrine tumors. (a) Hematoxylin and eosin staining: typically highly cellulated neuroendocrine tumor. (b) Hematoxylin and eosin staining: atypical neuroendocrine neoplasm with low cellularity and rich fibrous stroma
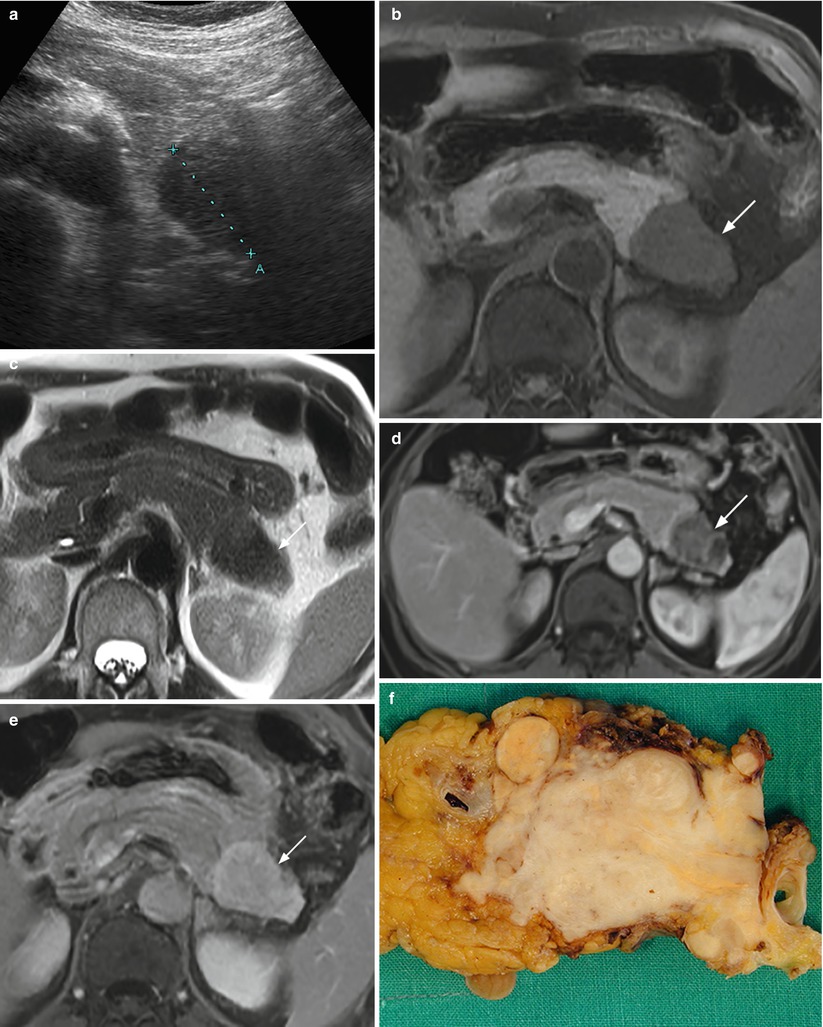
Fig. 36




Nonfunctioning fibrous neuroendocrine tumor. (a) Conventional US examination: hypoechoic lesion (calipers) with regular margins in the pancreatic tail. (b–e) MRI examination: the lesion appears hypointense both on T1-weighted fat-saturated axial images (arrow in b) and T2-weighted axial images (arrow in c). At dynamic MRI study, lesion is inhomogeneously hypovascular in respect to the surrounding pancreatic parenchyma in the postcontrastographic pancreatic phase (arrow in d), but with progressive intense contrast medium accumulation in the late phase (arrow in e). (f) Surgical specimen (distal pancreatectomy) of the fibrous neuroendocrine tumor
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree