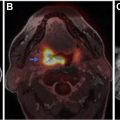
Neoplastic meningitis (NM) and paraneoplastic syndromes (PNSs) are a rare group of disorders present in patients with cancer. Clinical diagnosis of these conditions is challenging, and imaging and laboratory analysis play a significant role in diagnosing. Diagnosis of NM largely depends on documenting circulating tumor cells in the cerebrospinal fluid (CSF) and/or leptomeningeal and nodular enhancement on contrast-enhanced MR imaging of the brain or axial spine. PNSs encompass a variety of symptoms or syndromes. Paraneoplastic neuronal disorder diagnosis requires a multidimensional approach, high clinical suspicion, CSF and serum examination, and imaging. Neuroimaging is an integral part in the evaluation.
Key points
- •
Neoplastic meningitis (NM) is an uncommon metastatic manifestation of cancer, characterized by multifocal neurologic signs and symptoms.
- •
In a suspected case of NM, work-up includes detailed clinical examination, MR imaging of the brain and entire spine with and without contrast, cerebrospinal fluid analysis and pressure measurement, and systemic imaging, staging, and restaging of the primary tumor with PET–computed tomography or MR imaging.
- •
Paraneoplastic syndromes (PNSs) are a group of disorders present in patients with cancer, which may involve any part of the nervous system (central and peripheral nervous disorders) and simultaneously may affect multiple areas.
- •
PNSs encompass a variety of symptoms or syndromes, including limbic encephalitis, cerebellar degeneration, brainstem encephalitis, striatal encephalitis, and opsoclonus-myoclonus syndrome.
Introduction
Neoplastic meningitis (NM) is an uncommon metastatic manifestation of cancer, characterized by multifocal neurologic signs and symptoms and usually occurring late in the course of the disease. Diagnosis is challenging but essential to prevent progressive neurologic injury that substantially impairs cancer patients’ quality of life. Clinical examination has a minimal role in the diagnosis of NM. The most common tests employed are the brain and spinal axis MR imaging and cerebrospinal fluid (CSF) cytology.
The incidence of NM varies, depending on the primary site of the tumor. NM is diagnosed in 4% to 15% of patients with solid tumors (carcinomatous meningitis), 5% to 15% of patients with hematological malignancies (leukemic or lymphomatous meningitis), and 1% to 2% of patients with primary brain tumors. NM is associated with significant morbidity and short survival rates, ranging from several weeks to 8 months. Adenocarcinoma is the most frequent histology, whereas among the solid tumors, breast, lung, and skin are the most common primary sites of leptomeningeal metastasis. Although small cell lung carcinoma (SCLC) and melanoma have the highest rates of spread to the leptomeninges, with 11% and 20%, respectively, breast cancer, because of its higher incidence, accounts for most disorder cases despite a 5% metastatic rate. Lymphomatous meningitis is reported most commonly with diffuse high-grade non-Hodgkin lymphoma. Prophylactic treatment commonly is considered in patients with aggressive non-Hodgkin lymphoma, such as Burkitt lymphoma and lymphoblastic lymphoma, because these diseases have a greater than 25% risk of meningeal relapse without central nervous system (CNS)-directed therapy. NM is the initial manifestation of systemic cancer in only 5% to 10% of patients. Synchronous intraparenchymal brain metastases are evident in 11% to 31% of patients with NM.
Pathophysiology
For NM to occur, tumor cells must reach the meninges and CSF. The invasion of the meninges occurs through different pathways, depending on the histology of the primary tumor. These include hematogenous (venous or arterial) spread, neural spread (endoneuronal and perineural), perivascular lymphatic spread, and iatrogenic spread. , In addition, spread may occur directly from brain parenchyma or bony tumor lesions in the skull, spine, or choroid plexus or from de novo tumors. All routes give tumor cells access to the subarachnoid space of the CNS. With regard to hematogenous spread, tumor cells in the bloodstream become lodged in small-caliber CNS vessels, resulting in ischemia distal to the affected vessel. This disrupts vessel endothelium and surrounding basement membranes, allowing tumor cells to access the CSF around the damaged vessel (the Virchow-Robin space) and the subarachnoid space. , Tumor cells can gain access to the ventricular system by invading the subependymal lining of the ventricular wall.
The threshold number of tumor cells necessary to reach the CSF and result in clinical NM is unknown. The location of the tumor, however, plays a vital role in the spread. The closer proximity of the tumor to the meninges leads to a higher incidence of NM. Medulloblastomas (tumors located in or near the fourth ventricle) and pineoblastoma (near the pineal gland) are notable examples of this principle. Astrocytomas are not a significant cause of NM. Postoperative NM, presumably caused by intraoperative tumor cell seeding of the CSF, has been reported in 5% to 40% of patients after craniotomy. The risk of NM is higher in patients undergoing craniotomy for posterior fossa metastases than supratentorial metastases.
Clinical features
Clinical diagnosis of NM is challenging, and sufficient suspicion is required to warrant diagnostic testing. A majority of patients present with multifocal neurologic symptoms that vary according to anatomic areas of the CNS: cerebrum (15% of patients), cranial nerve/brainstem (35%), and spinal cord (60%) , ( Table 1 ). Cerebral symptoms include headache, dizziness/vertigo, confusion, fatigue, gait instability, aphasia, altered mental status, seizure, hemiparesis, and numbness. The most common of these manifestations are headache and mental status changes. , , When the posterior fossa is the primary site of the NM, most clinical symptoms are due to cerebellar dysfunction; these include unsteady gait, diplopia, ataxia, and falls. Cranial nerve lesions are reported in approximately 40% of patients. , , The most common dysfunctions are of the oculomotor, facial, cochlear, and optic nerves. Cranial nerve and brainstem symptoms include loss of visual acuity, diplopia, facial muscle weakness, hearing loss, dysphagia and dysarthria, hoarseness, decreased hearing, and facial pain or numbness. Diplopia is the most common symptom of cranial nerve dysfunction, with cranial nerve VI the most frequently affected, followed by cranial nerves III and IV. , , Symptoms involving the spinal cord may include lumbar pain, limb paresis or paralysis, bowel and bladder dysfunction, and loss of reflexes leading to cauda equine or cauda medullaris syndrome.
| Cerebral symptoms | Headache Mental changes Nausea Vertigo Vomiting Seizures Communicating hydrocephalus Gait alterations Coordination disorders |
| Cranial nerve dysfunction | Diplopia Vision loss Hearing loss Facial paresis Hoarseness Hypoacusia Ocular motility deficits Dysphagia |
| Spinal symptoms | Bilateral/unilateral pain Loss of reflexes Paresthesia Motor deficits Weakness in extremities |
One of the most common complications of NM is communicating hydrocephalus due to blockage of the basal cisterns and obstruction to CSF absorption at the arachnoid granulation level. , Less common complications are basal ganglia or internal capsule infarctions due to vascular compression and development of vasculitis, with vasospasm and thrombosis from exudate surrounding small perforating vessels. These are seen more commonly in pediatric patients.
Diagnosis
NM is diagnosed using the National Comprehensive Cancer Network guidelines. Diagnosis is established on positive CSF cytology for malignant cells, and/or leptomeningeal and nodular enhancement on computed tomography (CT) or MR imaging in a patient with known malignancy. There are ongoing efforts by Response Assessment in Neuro-Oncology (the Leptomeningeal Assessment in Neuro-Oncology scorecard) and the European Association of Neuro-Oncology–European Society of Medical Oncology to standardize the diagnostic work-up and interpretation of NM. , These groups use clinical symptoms, imaging, and CSF analysis for diagnosis and assessment of treatment. Unfortunately, neither of the 2 systems has been validated.
In a suspected case of NM, work-up includes detailed clinical examination, MR imaging of the brain and entire spine with and without contrast, CSF analysis and pressure measurement, and systemic imaging, staging, and restaging of the primary tumor with PET-CT or MR imaging. Although positive CSF cytology provides the highest degree of certainty in an NM diagnosis, it is not essential for initiating treatment if the remainder of the evaluation is supportive of NM.
Diagnosis of NM largely depends on documenting circulating tumor cells in the CSF. The site of the CSF tap and yield depend on the symptoms and type of the primary malignancy. Cytology of CSF obtained by lumbar puncture is more likely to be positive than CSF obtained from ventricles if spinal cord–related symptoms are present and vice versa if cranial-related symptoms are present.
CSF abnormalities include increased opening pressure (>200 mm of H 2 O), increased leukocytes (>4/mm 3 ), elevated protein (>50 mg/dL), and decreased glucose (<60 mg/dL). These findings may be suggestive of NM but do not confirm a diagnosis. , CSF cytology may be positive in only 45% of the cases on the first lumbar puncture, with an increase in yield up to 80% with a second CSF examination. Each subsequent lumbar puncture has a poor yield of only 2%. ,
Imaging plays a vital role and is the initial investigation of choice in a suspected case of NM. Contrast-enhanced (CE) MR imaging of brain ( Fig. 1 ) and axial skeleton remains the most sensitive imaging modality for diagnosis, with sensitivity of 76% and specificity of 77% in patients with supportive clinical findings. , Bacterial, fungal, or viral meningeal inflammation, neurosarcoidosis, chronic meningitis, and Guillain-Barré syndrome may mimic NM; therefore, it is important to correlate the imaging findings with the appropriate clinical setting.

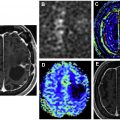
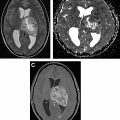
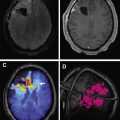
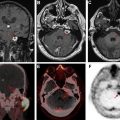
On MR imaging, noncontrast fluid-attenuated inversion recovery (FLAIR) images show hyperintensity within the sulci and cisterns due to increased CSF proteinaceous content ( Fig. 2 A). Contrast-enhanced T1-weighted imaging (T1WI) or magnetization-prepared rapid acquisition of gradient echo (MPRAGE) demonstrates subarachnoid, ventricular, or parenchymal enhancing nodules; focal or diffuse pial enhancement; and ependymal, sulcal, folial, or cranial nerve enhancement ( Fig. 2 B). , , Postcontrast 3-dimensional T2-FLAIR images provide higher sensitivity in detecting leptomeningeal abnormalities than conventional postcontrast T1 images or postcontrast MPRAGE. The most frequent brain MR imaging findings are subarachnoid nodules and pial enhancement. Due to dependency, enhancement commonly is appreciated around the basal cisterns and over the surface of the midbrain, pons, and over the cerebellar folia. Enlargement and enhancement of the cranial nerves are seen, most commonly within the oculomotor, facial, cochlear ( Fig. 3 ), and optic nerves ( Fig. 4 ). Most typically, enhancement is seen along the cisternal segments of the cranial nerves. Enhancement and dysfunction of cranial nerves III, IV, and VI are common causes of diplopia in patients with NM ( Fig. 5 ).




MR imaging also is sensitive for detecting metastatic deposits along the neuraxis. CE MR imaging of the spine shows smooth leptomeningeal enhancement or intradural extramedullary enhancing nodules, especially in the lumbar spine and over the cauda equine ( Fig. 6 ). Lumbosacral nerve root thickening and enhancement commonly is observed on CE MR imaging of the lumbar spine. Studies have suggested that MR imaging–proved leptomeningeal seeding is an indicator of poor prognosis and could be used to identify responses to intrathecal chemotherapy. Microscopic metastases, however, are below the resolution of MR imaging; therefore, cytology has a higher rate of specificity but a lower rate of sensitivity.

In highly suspected cases of neoplastic meningitis with negative CSF and imaging examination, other tests, such as CSF immunohistochemical examinations, polymerase chain reactions, fluorescence in situ hybridization, and cytogenetic analysis, can improve the detection rate. , Tumor-specific markers—such as carcinoembryonic antigen for adenocarcinomas, α-fetoprotein, and β-human chorionic gonadotropin for germ cell tumors; 5-hydroxyindoleacetic acid for carcinoid tumors; and immunoglobulins for multiple myeloma—are employed very rarely for the NM diagnosis. , Nonspecific tumor markers, such as vascular endothelial growth factor, creatine kinase–BB isoenzyme, tissue polypeptide antigen, and lactate dehydrogenase isoenzyme-5, can be strong indirect indicators of NM. None is sensitive enough, however, to improve on the cytologic diagnosis. In certain malignancies like leukemia or lymphoma, the sensitivity of flow cytometry is several degrees higher than that of cytology for detecting CSF leukemia or lymphoma.
Hydrocephalus remains one of the most common complications of the NM due to blockage of the basal cisterns and obstruction to CSF absorption at the arachnoid granulation level ( Fig. 7 ). , Placement of a ventriculoperitoneal shunt is an effective palliative approach in patients with symptomatic hydrocephalus. An Ommaya reservoir or similar devices, useful in administering intraventricular chemotherapy and CSF sampling, are employed routinely in NM patients. These devices are safer and more comfortable for the patient than repeated lumbar punctures and help in the uniform distribution of the drug in the CSF. , , It is crucial to ensure that the catheter’s tip and side perforations are inserted entirely into the ventricle to avoid drug instillation into the brain parenchyma. This placement needs to be confirmed with a CT scan to avoid chemotherapy related leukoencephalopathy. Other complications include reservoir malfunction and infection. Underdiagnosis and assessing response to treatment remain significant problems in NM patients when the CSF and imaging tests are negative.

Early diagnosis and treatment of NM are vital because most untreated patients die within 1 week to 9 weeks (median 3 weeks) due to neurologic disease and tumor progression. The main aim of treatment is to extend survival and stabilize or improve neurologic symptoms. To date, brain and neuraxis imaging and CSF analysis remain the backbone of investigations in a suspected case of NM.
Paraneoplastic syndromes
Paraneoplastic syndromes (PNSs) are a group of disorders that present in patients with cancer. PNSs may involve any part of the nervous system (central and peripheral nervous disorders) and may affect multiple areas simultaneously. PNS is a rare neurologic disorder seen approximately in 0.01% of cancer patients. PNS incidence largely depends on the tumor cell type. Approximately 30% of patients with thymoma have some form of neurologic autoimmunity, mostly myasthenia gravis, compared with pulmonary small cell carcinoma, which is associated with 1 or more PNSs in 3% of cases. , Other malignancies associated with PNSs include gynecologic malignancies arising from the breast, ovary, fallopian tube, and peritoneum; Hodgkin lymphoma and non-Hodgkin lymphoma; testicular cancer; and neuroblastoma. PNSs occur at a much lower rate in patients with larger cell lung, renal, uterine, and melanotic skin cancers.
PNSs encompass a variety of symptoms or syndromes, including limbic encephalitis, cerebellar degeneration, brainstem encephalitis, striatal encephalitis, and opsoclonus-myoclonus syndrome ( Box 1 ). , , Myelitis, motor neuron disease, stiff person syndrome, Lambert-Eaton myasthenic syndrome (LEMS), neuromyotonia, and Guillain-Barré syndrome also are included. Manifestations precede the diagnosis of cancer in many cases.
Brain
Paraneoplastic encephalomyelitis
Limbic encephalitis
Brainstem encephalitis
Opsoclonus-myoclonus
PCD
Chorea
Eye
Paraneoplastic optic neuritis
Paraneoplastic retinal degeneration
Spinal cord
Myelopathy
Myelitis with rigidity and spasms
Motor neuronopathy
Acute necrotizing myelopathy
Nerves
Sensory neuronopathy
Sensorimotor peripheral neuropathy
Autonomic neuropathy, gastrointestinal dysmotility
Motor neuronopathy
Neuromuscular junction/muscle
LEMS
Myasthenia gravis
Dermatomyositis
Neuromyotonia
Muscle
Polymyositis/dermatomyositis
Acute necrotizing myopathy
Multifocal disorders
Encephalomyeloneuropathies
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree