Nephrocalcinosis and Nephrolithiasis
Intrarenal calcifications may lie in the renal parenchyma (nephrocalcinosis) or collecting system (nephrolithiasis). Dystrophic calcification is calcification of abnormal tissue such as tumors, cyst walls, inflammatory masses, or vessels. Because dystrophic calcification is caused by the underlying parenchymal abnormality, it is not considered nephrocalcinosis.
Nephrolithiasis is stone formation within the collecting system. Most stones are formed in the pelvocalyceal system and may be passed distally. Occasionally, stones may form in a cavity that communicates with the collecting system, such as a calyceal diverticulum, in the bladder, or a urethral diverticulum. Those conditions that result in nephrocalcinosis often lead to nephrolithiasis.
Because calcification is readily detected on an abdominal radiograph, nephrocalcinosis and nephrolithiasis may be evaluated with conventional urography. When supplemented with computed tomography (CT), even relatively lucent stones are easily detected. Because of its ability to detect nonurologic causes of abdominal pain, CT has become the primary imaging modality for evaluating patients with suspected acute ureteral obstruction from a ureteral calculus.
▪ NEPHROCALCINOSIS
Calcification of abnormal tissue is termed dystrophic. Dystrophic calcification may occur when the solubility product of calcium and phosphate is exceeded because of a change in pH, or may be part of a reparative process. This may occur in a large variety of lesions, but the most common are tumor, hematoma, an inflammatory mass, and vascular abnormalities. Dystrophic calcifications are considered in the discussion of the specific entities.
Calcification of normal renal tissue due to abnormally high levels of serum calcium is termed metastatic. Metastatic calcification occurs when the solubility product of calcium and phosphate or oxalate in the extracellular fluid is exceeded. Certain diseases have a predilection to calcify specific anatomic areas (Table 13.1). Thus, metastatic nephrocalcinosis may be further subdivided by the predominant location.
Cortical Nephrocalcinosis
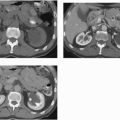
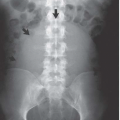
Cortical nephrocalcinosis is located in the periphery and along the central septa of Bertin. The medullary pyramids are spared. Cortical nephrocalcinosis may be seen on abdominal radiographs as thin peripheral lines of calcification (tram lines), diffusely dense renal shadows, or as diffuse punctate calcifications representing necrotic cortical tubules (Fig. 13.1). The cortex is echogenic on ultrasound, but typically does not have shadowing, and noncontrast CT shows high attenuation of the cortex.
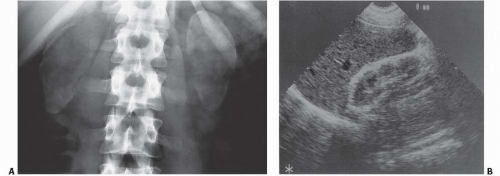
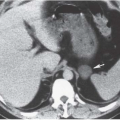
The most common entities to produce cortical nephrocalcinosis are chronic glomerulonephritis, acute cortical necrosis, and oxalosis. Cortical nephrocalcinosis may also be seen in Alport syndrome (hereditary nephropathy and deafness) (Fig. 13.2) and in patients with a rejected renal transplant. Infection with Pneumocystis carinii, Mycobacterium avium-intracellulare (MAI), and cytomegalovirus can result in multiple stippled calcifications in the renal cortex in patients with AIDS.
Acute cortical necrosis may be caused by ingestion of toxins such as ethylene glycol, exposure to methoxyflurane anesthesia, or an acute vascular insult. Both ethylene glycol and methoxyflurane exposure result in oxalate deposition that causes interstitial fibrosis. Acute hypotension may cause focal tubular necrosis that later calcifies; one typical clinical situation during pregnancy is placenta abruptio with hemorrhage.
Hereditary nephritis, or Alport syndrome, is characterized by glomerulonephritis and interstitial fibrosis, and is frequently associated with nerve deafness. It is inherited as an autosomal-dominant trait with variable penetrance. The disease is transmitted to both sons and daughters, but it is incompletely expressed in women. Hematuria often begins in childhood and mild proteinuria may be present. Progression to renal failure is slow, with death by the third to fifth decade.
MAJOR CAUSES OF CORTICAL NEPHROCALCINOSIS
Chronic glomerulonephritis
Acute cortical necrosis
Oxalosis
Medullary Nephrocalcinosis
Medullary nephrocalcinosis is central in location and spares the cortex. It is usually a bilateral process with multiple stippled calcifications in the characteristic medullary distribution (Fig. 13.3); the exception is medullary sponge kidney, which may be unilateral or segmental. On ultrasound, the pyramids are echogenic and may or may not have shadowing; depending on the size of the
calcifications, CT clearly shows the high-attenuation calcifications in the medulla.
calcifications, CT clearly shows the high-attenuation calcifications in the medulla.
TABLE 13.1 Causes of Hypercalciuria | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
MAJOR CAUSES OF MEDULLARY NEPHROCALCINOSIS
Renal tubular acidosis (RTA) type I (distal)
Hypercalcemic states
Medullary sponge kidney
The most common etiologies to produce medullary nephrocalcinosis are hyperparathyroidism and RTA. Other relatively common causes include tubular ectasia (medullary sponge kidney), the milk-alkali syndrome, sarcoidosis, vitamin D intoxication, and a variety of nephrotoxic drugs such as amphotericin B.
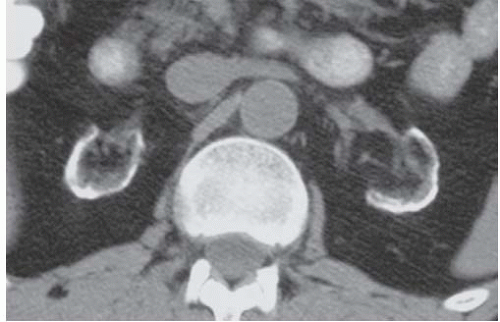 FIGURE 13.2. Cortical nephrocalcinosis. CT scan of a patient with Alport syndrome and cortical nephrocalcinosis. |
Hyperparathyroidism
Hyperparathyroidism may be caused by a parathyroid adenoma, carcinoma, or hyperplasia of the chief cells. It is most commonly due to a single adenoma. Typically, the serum calcium level is high and the phosphate level is low; this helps distinguish primary hyperparathyroidism from secondary hyperparathyroidism, resulting from chronic renal disease in which the serum phosphate is elevated.
Renal Tubular Acidosis
Patients with renal tubular acidosis (RTA) have a defect in the tubules that prevents the kidney from excreting an acid urine. Thus, the risk of stone formation is increased, as calcium salts are less soluble in alkaline urine than in acid urine. In adults, primary RTA is caused by an inherited enzymatic defect. The manifestations of urolithiasis, osteomalacia, and hypokalemia can be treated with alkalinizing salts. Secondary RTA occurs as a result of a diminished ability of the distal tubule to excrete hydrogen ions. Disease processes that cause secondary RTA by impairing hydrogen ion excretion by the distal tubule include Fanconi syndrome, Wilson disease, and amphotericin B toxicity.
RTA is divided into proximal and distal forms. The proximal variety (type II) of RTA does not have radiographic manifestations. In the distal form (type I), the distal renal tubule can no longer secrete hydrogen ions. This results in bicarbonate loss, reduced acid excretion, secondary aldosteronism, and hypokalemia. Nephrocalcinosis occurs in approximately 75% of these patients and commonly appears as dense clusters of calcifications in the medullary pyramids.
Milk-Alkali Syndrome
Nephrocalcinosis may occur in patients who consume large quantities of antacids and milk for the treatment of peptic ulcer disease. This has been termed the milk-alkali syndrome. Alkaline urine facilitates precipitation of calcium-containing calculi.
Medullary Sponge Kidney
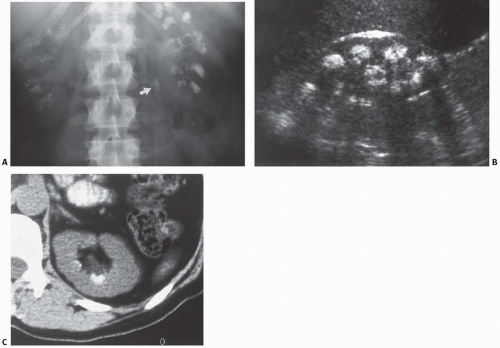
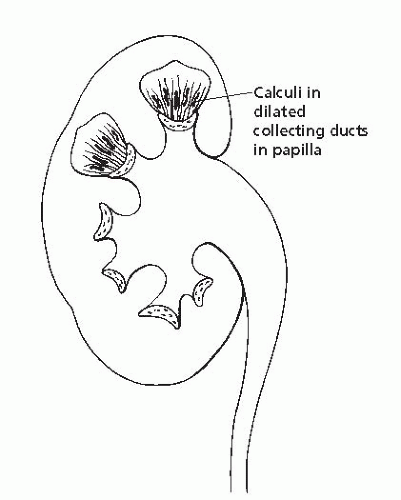
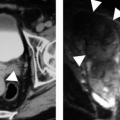
The stones found in medullary sponge kidney are calcium phosphate or calcium oxalate. They are usually small, and many more are present than can be detected radiographically. These tiny stones are asymptomatic, but may cause colic if they migrate into the collecting system, enlarge, and begin to pass down the ureter. This condition is caused by cystic dilation of the distal collecting ducts, which causes stasis of urine and stone formation. Because this is an anatomic rather than a metabolic defect, it can occur unilaterally or even segmentally in one or both kidneys. This type of distribution should suggest the diagnosis, as should a linear configuration of medullary calculi. Further evidence is pooling of contrast around the stones in the dilated collecting ducts during urography (Figs. 13.4 and 13.5).
▪ NEPHROLITHIASIS
Nephrolithiasis, also known as urolithiasis, is a common problem among people from temperate climates. Interestingly, a low urinary output is believed to aid in urinary tract stone formation, yet people from areas with hot climates such as Africa have a low incidence of stone disease. Perhaps they are genetically less likely to form stones. In the United States, the southeastern states often are called the stone belt because they have the highest incidence of stone disease. Hospital admissions for stone-related problems are twice as common in the southeast as the rest of the nation.
There are many etiologies of nephrolithiasis including genetic predisposition, diet, occupation, and lifestyle. A high water intake resulting in an increased urinary output significantly reduces the incidence of stones in patients predisposed to stone formation.
Nephrolithiasis is seen three times as often in men as in women. The peak age for the onset of renal stone disease is 20 to 30 years, but the tendency for stone formation is often lifelong. Patients treated for nephrolithiasis are most commonly between 30 and 60 years.
The most common presenting symptom is renal colic, which is usually caused by an obstructing ureteral stone. Controversy remains regarding whether calyceal stones cause pain. Most calyceal stones are asymptomatic, but occasionally their removal is associated with relief of pain.
Renal colic is abrupt in onset, most frequently begins in the flank, and radiates to the groin. Men may complain of testicular pain, whereas women feel discomfort radiating to the labia majora. Typically, patients suffering renal colic cannot find a comfortable position and continue to move around. Patients with stones in the distal ureter may present with symptoms of bladder irritability.
Hematuria is present in the vast majority of patients with urolithiasis, but may be absent, particularly if the stone is completely obstructing. Pyuria is uncommon, but a culture should be obtained to detect a urinary tract infection. Bove et al., in a study using CT as the standard for stone detection, found that 33% of their patients with documented ureteral stones had five or fewer red cells per high-powered field on microscopic examination; 11% had no hematuria whatsoever.
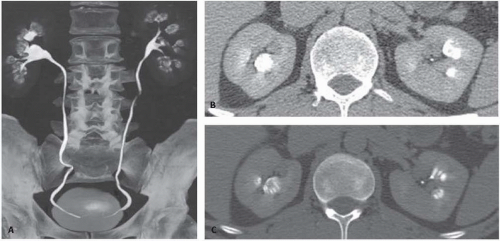 FIGURE 13.5. Medullary sponge kidney. CT Urogram image (A) and two axial images (B, C) from a CTU in a patient with medullary sponge kidney. Dilated distal collecting ducts are present. |
Specific Types of Urolithiasis
Calcium Stones
Calcium oxalate and calcium phosphate stones crystallize when the product of the two ions exceeds the solubility product. Thus, both the amount of calcium and the amount of oxalate are important in determining stone formation. A discussion of these factors is beyond the scope of this book, but factors affecting hypercalciuria are presented in Table 13.1.
Hypercalcemia is a common cause of hypercalciuria, although it is present in a minority of patients, and may result from a variety of metabolic processes. An abnormally large amount of calcium may be absorbed from the digestive tract. This occurs with hypervitaminosis D, sarcoidosis, and the milk-alkali syndrome. Too much calcium may be mobilized from the bony skeleton. This may result from immobilization, extensive bone metastases, or hyperparathyroidism.
Ionized calcium is filtered by the glomerulus, but most of this free calcium is reabsorbed by the tubules. However, there is a maximum reabsorption that can occur. If this is exceeded by a high calcium load in patients with hypercalcemia, hypercalciuria will result. Hypercalciuria may also be idiopathic; this condition is probably the most common cause of nephrolithiasis.
Calcium stones are relatively dense and are usually easily seen on abdominal radiographs. Although the large majority of urinary calculi are radiodense (approximately 85%), the remainder are radiolucent and not seen on plain films. The incidence of urinary stones by chemical composition is given in Table 13.2.
Cystine Stones
Patients with cystinuria have a defect in renal tubular reabsorption of the amino acids cystine, ornithine, lysine, and arginine. The defect is inherited as an autosomal-recessive trait, and is present in the intestinal mucosa as well as in the renal tubular cells. The only manifestation in most patients is nephrolithiasis. Excess cystine excreted in the urine exceeds its solubility, and cystine stones are produced.
The opacity of cystine stones depends on how much contamination with calcium is present. However, many stones are pure cystine and are still easily seen on abdominal radiographs, although they are not as dense as calcium stones.
TABLE 13.2 Incidencea of Urinary Lithiasis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Infection Stones
Magnesium ammonium phosphate (struvite) stones form when the urine pH is more than 7.2. They commonly occur in the setting of a urinary tract infection with a urea-splitting, gram-negative enteric organism, often Proteus mirabilis. Thus, struvite calculi are also commonly referred to as infection stones. Because women have more urinary tract infections, struvite stones are seen more frequently in women than in men.
COMPOSITION OF INFECTION STONES
Struvite (magnesium ammonium phosphate) + apatite (calcium phosphate) = triple phosphate (calcium-magnesium-ammonium phosphate)
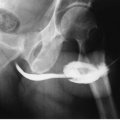
Pure struvite stones are essentially radiolucent and rare. Typically, struvite is found mixed with calcium phosphate (apatite) to form the so-called triple phosphate stones, which are easily seen on plain radiographs. These calcium-magnesium-ammonium phosphate stones are seen in association with infected urine; to refer to them as struvite alone is a misnomer. They account for approximately 70% of staghorn calculi (Fig. 13.6), the remainder being composed of cystine or uric acid.
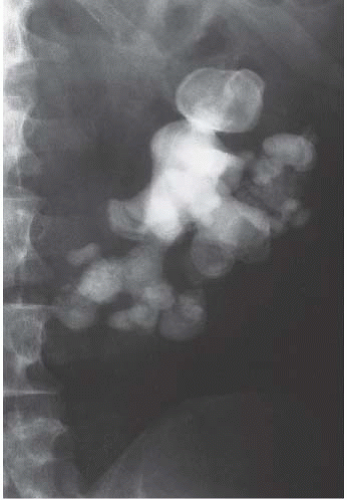 FIGURE 13.6. Staghorn calculus. A large staghorn calculus is easily seen in the left kidney. The alignment suggests a horseshoe kidney. |
STAGHORN CALCULI
Triple phosphate (calcium-magnesium-ammonium phosphate)
Uric acid
Cystine
Uric Acid Stones
Unlike other mammals, humans lack the enzyme uricase, which converts uric acid into allantoin. In the urine, uric acid exists either free or as the much more soluble salt, sodium urate. Acid urine contributes to an increased concentration of the less soluble free uric acid.
Patients with urate calculi have hyperuricosuria, but do not necessarily have hyperuricemia. Idiopathic uric acid lithiasis occurs in patients with normal serum urate levels, but with a persistently low urine pH. This is seen in patients who take medications to acidify their urine, but it may also be present in patients with chronic diarrhea or an ileostomy.
Inborn errors of metabolism may result in hyperuricemia and uric acid lithiasis. Patients with gout or Lesch-Nyhan syndrome are prone to form uric acid stones. Similarly, overindulgence in foods high in purine and proteins metabolized to uric acid may lead to hyperuricemia, hyperuricosuria, and uric acid stones. The ingestion of uricosuric drugs such as salicylates and thiazides may increase the urine uric acid concentration sufficiently to allow uric acid stone formation.
In the United States, uric acid stones account for 5% to 10% of renal stones. The reported incidence varies by country; in Israel, approximately 75% of renal stones are uric acid stones.
Uric acid stones are insufficiently radiopaque to be seen on an abdominal radiograph. They account for the majority of “lucent” stones; however, they are still sufficiently dense to be easily seen on CT and produce shadowing on ultrasound.
Xanthine Stones
These very rare stones may be seen in patients with hereditary xanthinuria, but may also be present in patients treated with allopurinol, which blocks the conversion of xanthine to uric acid. Xanthine stones are relatively radiolucent because their density is similar to uric acid stones.
Matrix Stones
Matrix stones are composed primarily of coagulated mucoids with very little crystalline component. They are found most commonly in patients with urease-producing infections such as those caused by Proteus species. Matrix stones are also relatively radiolucent and may be confused with uric acid calculi. However, matrix stones occur in the presence of an alkaline urine, whereas the urine is acidic in patients with uric acid calculi.
STONES RADIOLUCENT ON PLAIN RADIOGRAPHS
Uric acid
Xanthine
Matrix
Pure struvite
Protease inhibitor
Protease Inhibitor Stones
Protease inhibitors such as indinavir are effective in treating patients with human immunodeficiency virus type I (HIV-1) infection. However, the drug is partially excreted in the urine, where it has a low solubility and is prone to precipitate out as crystals. Indinavir crystals are flat, rectangular plates that may be found in the urine in a variety of patterns. They are radiolucent on either abdominal
radiographs or CT. Over time, these crystals may calcify and become dense enough to be seen on CT or an abdominal radiograph.
radiographs or CT. Over time, these crystals may calcify and become dense enough to be seen on CT or an abdominal radiograph.