Prostate and Seminal Vesicles
▪ PROSTATE
Benign Prostatic Hypertrophy
The term benign prostatic hypertrophy (BPH) refers to hyperplasia of the glands in the transitional zone, superior to the verumontanum.
Etiology
Several factors influence the development of prostatic hypertrophy. Testosterone stimulates prostatic growth, as evidenced by the fact that eunuchs never develop prostatic hypertrophy and that exogenous testosterone causes an increase in the size of the prostate of castrated patients. Estrogen administered to the uncastrated male causes prostatic atrophy; administration of progesterone to patients with obstructive voiding symptoms due to prostate hypertrophy who are poor surgical risks results in relief of symptoms in 50% of patients. Paradoxically, prostatic hypertrophy occurs in the setting of aging, gonadal involution, and a consequent reduction in the production of testosterone. Hepatic cirrhosis is often accompanied by prostatic atrophy.
Incidence
The prostate gland in adult males younger than 50 years is about the size of a walnut and weighs 15 to 20 g. After 50 years of age, 50% of men have some degree of prostatic hypertrophy. The prostate may double in size before the age of 70 years, and in some elderly men, can weigh several hundred grams. However, only approximately 10% of men require medical or surgical intervention.
Clinical Presentation
Enlargement of the prostate often results in obstructive voiding symptoms. However, the correlation between the degree of prostatic enlargement and the presence or severity of symptoms is weak.
The complex of voiding symptoms due to bladder outlet obstruction from any cause is known as prostatism; although BPH is the most common cause, prostate cancer, bladder neck contracture, or urethral stricture can also be responsible. The initial symptom is most commonly a reduction in force of the urine stream. Nocturia is common and is associated with a feeling of necessity to continue voiding even after voiding has ceased. Difficulty in initiating urination (hesitancy, straining) may have been present for some time before the patient seeks medical advice. Urinary retention may be of gradual onset or may occur suddenly. Although sudden acute retention is often an indication for prostate surgery, 50% of patients with acute retention are relieved by catheter drainage and may subsequently void relatively well for a period before requiring surgery.
Radiology
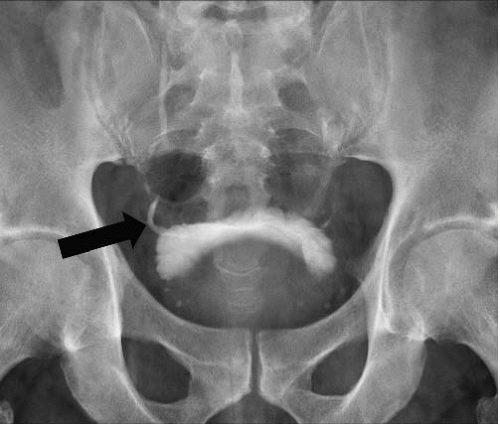
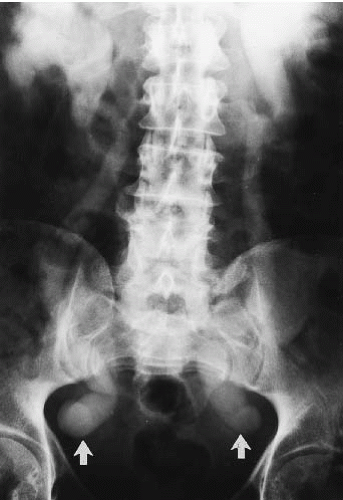
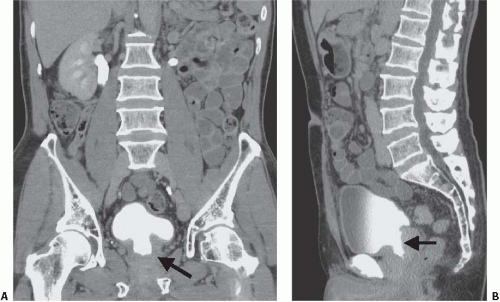
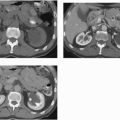
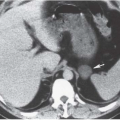
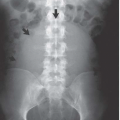
In young men, the prostate gland measures about 3 cm in diameter on axial images. Although computed tomography (CT) usually shows the prostate as a homogeneous organ, in a minority of contrast-enhanced studies, the posterior peripheral zone can be seen as an area of relatively lower attenuation than the centrally located adenoma. As the prostate enlarges (Fig. 16.1), evidence of outlet obstruction may increase. Findings include poor emptying of the bladder, varying degrees of thickening and trabeculation of the bladder wall and indentation of the bladder base (Fig. 16.2). The enlarged prostate elevates the interureteric ridge, producing a characteristic J shape or “hooking” of the distal ureters (Fig. 16.2). When detrusor hypertrophy can no longer compensate for bladder outlet obstruction, the bladder begins to dilate. Bladder diverticula result from long-standing outlet obstruction, may be larger than the bladder itself, and may actually deviate the bladder. In such cases, the diverticulum is smooth walled and the bladder has a thick, irregular (trabeculated) wall (Fig. 16.3). Bladder stones may also occur from urinary retention resulting from long-standing outlet obstruction by prostatic hypertrophy.
Other radiographic patterns can be seen with prostate hypertrophy. The so-called median lobe (Fig. 16.4), by virtue of a relatively narrow attachment to the prostate proper, can prolapse into the bladder neck and produce obstruction during voiding. Enlargement of this portion of the gland is more closely related to risk of urine retention than is overall prostate volume. The median lobe can look like a large, rounded filling defect in the center of the bladder on excretory urography or cystography but CT or ultrasound clearly show that the mass is contiguous with more inferior prostatic tissue.
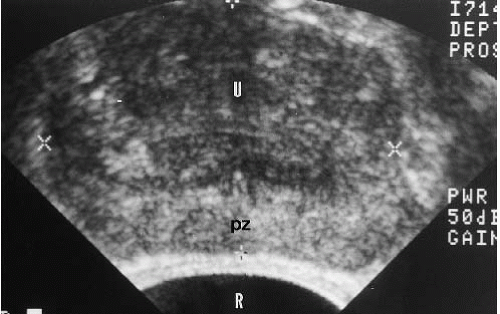
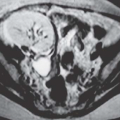
With long-standing severe bladder outlet obstruction, the upper urinary tracts can become dilated because of high-intravesical pressure. Contrast excretion is delayed, and renal function may be impaired. Typically, there is bilateral symmetric ureteropyelocaliectasis, in which the ureters are dilated to the level of the ureterovesical junctions (Fig. 16.5). Bladder outlet obstruction almost never causes unilateral hydronephrosis; conversely, bilateral symmetrical hydronephrosis is much more often produced by a high-pressure bladder than by bilateral ureteral lesions. Transrectal ultrasound (TRUS) can demonstrate significant prostatic enlargement, bladder calculi, and residual urine. TRUS often permits definition of the internal glandular structure, and prostatic adenomata arising in the transitional zone and periurethral glandular tissue are well visualized (Fig. 16.6). The transitional zone and periurethral glandular tissue are normally coarser and slightly less echogenic than tissue in the peripheral zone. Adenomata arising from
the transitional zone vary in echogenicity but are more commonly hypoechoic or show mixed echogenicity.
the transitional zone vary in echogenicity but are more commonly hypoechoic or show mixed echogenicity.
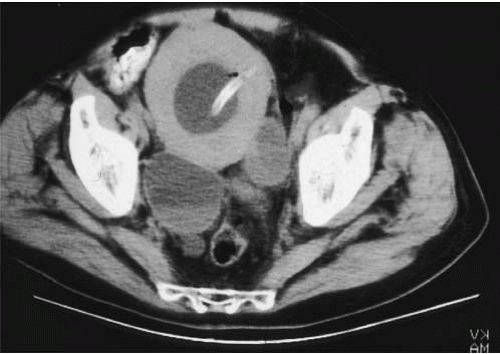
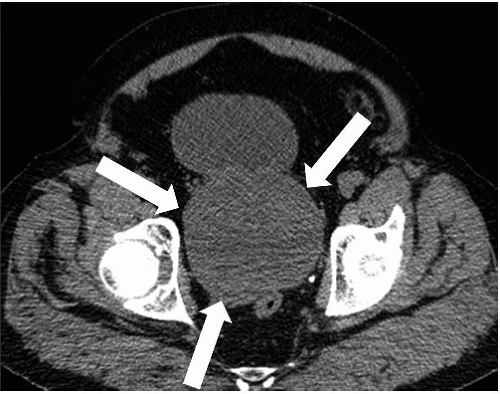 FIGURE 16.1. BPH. CT shows markedly enlarged prostate (arrows); a portion of the bladder is seen anterior to the prostate. |
Treatment of BPH
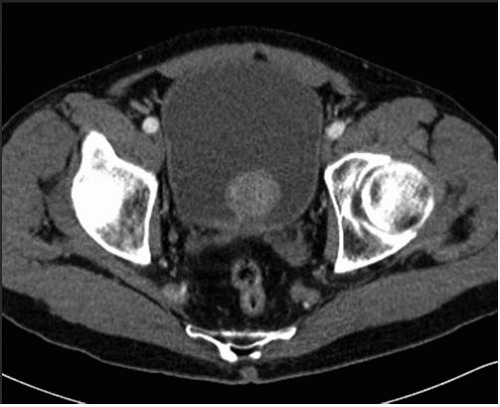
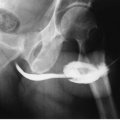
In many cases, symptoms related to benign prostatic enlargement respond well to oral medication, including alpha blockers like doxazosin and 5-a-reductase inhibitors like finasteride. However, persistent symptoms may require surgical intervention. For obstruction due to moderate enlargement of the prostate, transurethral resection of the prostate (TURP) is usually performed. This involves removal of the bladder neck and prostatic tissue surrounding the urethra through a resectoscope. With the internal sphincter at the bladder neck removed, urinary continence is maintained by the external sphincter surrounding the membranous urethra. During imaging of the bladder and urethra, the resected area of the prostate can be easily visualized because it is filled with urine (Fig. 16.7); this is known as a TURP defect. For more significantly enlarged glands, an open prostatectomy is employed; in this procedure, the prostatic capsule is opened and the obstructing adenoma is enucleated.
Prostate Cancer
Incidence
Prostate cancer is a very large public health problem in the United States; there are expected to be nearly a quarter of a million new cases diagnosed this year with over 30,000 deaths. Prostate cancer is
the most common internal malignancy among American men, and the second most common cause of death from tumor.
the most common internal malignancy among American men, and the second most common cause of death from tumor.
Prostate cancer is rare in men younger than 50 years but increases dramatically with age thereafter. Significant geographic and racial differences in the incidence of prostate cancer are reported. The incidence of clinical carcinoma is low in China, Japan, Hispanic areas, Israel, Latin and South America, and in Native Americans. The incidence is high in North America and Northern Europe. North American blacks have an incidence approximately twice that of whites. However, the incidence of occult carcinoma is uniform throughout the world. The geographic incidence may be influenced by the lack of epidemiologic studies in low-incidence countries and by the lack of documentation of prostate cancer. Prostatic carcinoma, like prostatic hypertrophy, is androgen dependent. Castrated males do not develop prostate cancer.
Pathology
More than 95% of prostatic neoplasms are adenocarcinomas, which typically originate in the epithelium of prostatic acini in the
peripheral zone of the prostate. Other prostate tumors are rare but include squamous cell carcinomas, endometrioid carcinomas arising from the prostatic utricle, carcinosarcomas, melanomas and mesenchymal neoplasms such as rhabdomyosarcoma, leiomyosarcoma, or fibrosarcoma. The remainder of this discussion pertains to adenocarcinoma of the prostate.
peripheral zone of the prostate. Other prostate tumors are rare but include squamous cell carcinomas, endometrioid carcinomas arising from the prostatic utricle, carcinosarcomas, melanomas and mesenchymal neoplasms such as rhabdomyosarcoma, leiomyosarcoma, or fibrosarcoma. The remainder of this discussion pertains to adenocarcinoma of the prostate.
The Gleason system for grading prostate cancer involves a histologic evaluation of tissue for glandular pattern, size, and distribution; cells are examined for their degree of differentiation. Predominant and secondary patterns of tumor are identified; each of these is assigned a score of 1 through 5 and the two added to create an overall score ranging from 2 to 10. The higher the grade, the less differentiation is present. Correlation between tumor grade and degree of spread (stage) is high; there are also strong correlations between Gleason scores and prognosis.
Staging
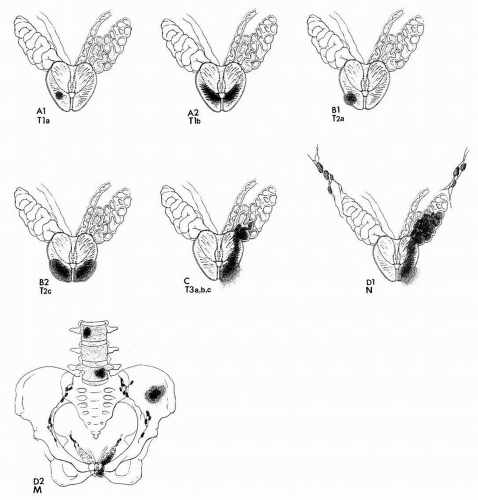
The Whitmore-Jewett staging method has been commonly used for staging prostate cancer, but modern practice more frequently uses the TNM system (Fig. 16.8 and Table 16.1). Advanced local
stage confers risk both for synchronous metastatic disease and for progression after treatment.
stage confers risk both for synchronous metastatic disease and for progression after treatment.
TABLE 16.1 Comparison of Staging Systems for Prostate Cancer | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Screening for Prostate Cancer
Detection of prostate cancer is performed by digital rectal examination (DRE) and measurement of prostate-specific antigen (PSA). PSA is a serine protease that is normally secreted only into the ductal system of the prostate but is often elevated in patients with prostate cancer. Derangement of the prostate architecture due to various conditions can cause PSA to leak from the acini into the stroma of the gland and then to enter the circulation through lymphatics and capillaries. There are multiple causes for an elevated PSA level, including recent rectal examination of the prostate and interventions such as cystoscopy, core needle biopsy of the prostate, and TURP. Elevated PSA levels can also be found in association with prostatitis, prostatic infarct, acute urinary retention, and benign hypertrophy of the prostate.
Serum PSA was originally thought to have an upper limit of normal of 4.0 ng per mL; more recently, it has been found that men with levels between 2.5 and 4.0 may harbor tumor. All other things being equal, the higher the PSA level the higher the risk that the patient will have prostate cancer, but there are a number of confounding factors: advancing age, BPH, and prostatitis can raise the level in the absence of tumor. Dividing the serum PSA level by the volume of the prostate (usually determined by TRUS) yields a parameter known as PSA density, which may more reliably screen for tumor. The rate of increase of PSA over time (PSA velocity) is felt to be a better indicator of tumor likelihood than a single PSA determination. Serum PSA is either free or bound to plasma proteins. A higher proportion of PSA is bound to proteins in patients with prostate cancer, resulting in a decrease in the ratio of free PSA to total (free plus protein-bound) PSA in such patients.
Screening with DRE significantly depends on the skill of the examiner. It detects only approximately 40% of prostate cancers less than 1.5 cm in diameter. Further, more than 50% of prostate cancers are clinically advanced at the time they are detected by DRE. Neither DRE nor PSA is highly sensitive; they may be used simultaneously in screening. If both DRE and PSA are positive, 60% of those patients will have prostate cancer; if both DRE and PSA are normal, only 2% of those patients will have the disease.
Screening for prostate cancer remains controversial because the natural history of the small tumors detected by elevated PSA levels is difficult to predict. The discrepancy between the diagnosis and death rates, and the high prevalence of asymptomatic cancers in autopsy studies, make it clear that many cancers are clinically insignificant and do not need treatment. Furthermore, the treatments for localized cancer, which include surgery, brachytherapy, external beam radiotherapy and local ablative measures like cryotherapy and high-intensity focused ultrasound, are not without side effects, including erectile dysfunction, urinary incontinence, fistulae and proctitis. A recent report of the U.S. Preventive Services Task Force has not supported generalized screening for these reasons. Nevertheless, when prostate cancers are confined to the prostate gland at the time of discovery, more than 85% are curable, and it is felt that at least some of these would progress and shorten life spans if not treated. Both the American Cancer Society and the American Urological Association recommend an annual DRE and PSA beginning at 50 years of age. For high-risk patients such as black men and those with a strong family history of prostate cancer, this screening is recommended to begin at 40 years of age.
No imaging modality is used for screening for prostate cancer. Ultrasound, CT, magnetic resonance imaging (MRI), scintigraphy and positron emission tomography (PET) are insufficiently accurate and/or too expensive to use for screening.
Dissemination
Carcinoma of the prostate typically originates in the peripheral zone, closer to the prostatic capsule than to the urethra. Spread of prostatic carcinoma occurs by three methods: direct extension, lymphatic spread, and hematogenous spread. Capsular invasion
opens a route to perineural lymphatics and to the periprostatic venous plexus of Santorini, allowing distant metastases to the viscera and axial skeleton.
opens a route to perineural lymphatics and to the periprostatic venous plexus of Santorini, allowing distant metastases to the viscera and axial skeleton.
Local spread is to the seminal vesicles, the urethra, the bladder neck, the bladder base, and the interureteric ridge, the last causing asymmetric or unilateral ureteral obstruction. Less commonly, prostatic cancer spreads posteriorly and superiorly, invades Denonvilliers fascia, and grows into the rectosigmoid region of the colon, sometimes producing bowel obstruction. Rarely, rectal involvement produces an ulcerating lesion that is extremely difficult to distinguish from rectal carcinoma. Late local spread to the corpus cavernosa, corpus spongiosum, and scrotum has been reported.
Lymphatic spread is initially from lymphatics within the prostate to the pelvic lymph nodes, which may be dissected for frozen section histologic examination at the time of total prostatectomy (staging lymphadenectomy). There is a high incidence of pelvic lymph node metastases in carcinoma of the prostate. The lymph node groups most commonly involved include the obturator, the external iliac, and the internal iliac nodes. The common iliac, paraaortic, mediastinal, and supraclavicular lymph nodes may be involved in advanced disease but are almost invariably associated with disease in the primary drainage sites.
Hematogenous spread is to the axial skeleton or viscera. Eighty-five percent of patients dying of prostatic carcinoma exhibit bony metastases in the axial skeleton. Sites of skeletal metastases are the lumbar spine, proximal femur, bony pelvis, thoracic spine, ribs, sternum, skull, and proximal humerus in order of decreasing frequency. The route of early hematogenous spread to the spine and pelvis is through the intervertebral venous plexus of Batson, which directly communicates with the periprostatic venous plexus of Santorini. More than 90% of prostatic metastatic lesions to bone are blastic. Spread to organs other than nodes and bones is uncommon except in patients with widely disseminated disease.
Nomograms, known as the Partin tables, which combine clinical stage (determined by the DRE), serum PSA levels, and the Gleason grade in the biopsy specimen, have been developed to predict the pathologic stage of the cancer as determined from examination of surgical specimens.
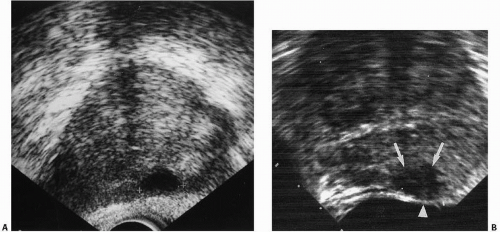 FIGURES 16.9. A, B Two cases of prostate carcinoma. TRUS images reveal them as marked (cursors in A, arrows in B) hypoechoic peripheral zone regions. |
Diagnosis
Once prostate cancer is suspected based on the DRE, PSA, or both, biopsy is usually performed; even if DRE, PSA, and radiologic discovery of widespread metastatic disease makes the diagnosis all but certain, reliable diagnosis requires tissue. A spring-loaded biopsy gun, guided by TRUS, obtains cores of prostate tissue and can be performed via a transrectal or transperineal route. The commonest method is to obtain multiple cores from representative portions of the gland. Multiple cores are routinely taken, sometimes as many as several dozen (“saturation” biopsy) may be obtained. As tumors may be small, obviously the more biopsies are performed the more likely these small tumors are to be diagnosed. TRUS can also be used to guide biopsy needles to hypoechoic regions in the prostate, which are especially likely to contain tumor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree