Fig. 1.1
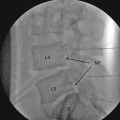
Dual Octrode leads for INRS (a) The left, lateral lead (arrow) in this case overlies the entering fibers of C5, C6, and C7 for treatment of C6 dermatome central pain in an MS patient. The more medial lead guards the lateral lead to isolate stimulation over the dorsal root entry zone at C6. On the lateral view (b), the lateral lead is positioned immediately at the dorsal border of the neural foraminae. The more medial lead rises dorsally over the convexity of the spinal cord as it courses rostrally toward the dorsal columns [53]
Selective nerve root stimulation (SNRS) involves targeting the dorsal root of the spinal nerve at the neural foramen through an intra- or extraspinal approach where the electrode lies in a parallel with the entering fibers. We include sacral nerve stimulation in this category. SNRS accomplishes the goal of capturing paresthesia in some difficult-to-treat lumbosacral segments where traditional methods fail. A cephalocaudal (retrograde) lateral epidural approach at L2/3 below the conus was developed to facilitate placement of the electrode “in line” with lumbosacral roots. Using this technique, a quadripolar electrode enters at midline and is rotated toward but not into the L4 foramen. The distal contact is commonly programmed as an anode and the three proximal contacts as cathodes. Appropriately positioned, one may capture the L4, 5 and S1 roots with a single lead. Retrograde cervicothoracic electrode placements have not been performed due to the risk of cord injury.
S2–4 roots can be captured by directing the quadrupole toward but not through the S2 foramen. For sacral neuromodulation, trial leads are commonly placed through the caudal sacral hiatus and advanced over the lumbosacral nerve roots of interest; if successful, a surgically implanted paddle lead may be placed via laminotomy. Conditions treated with this approach include interstitial cystitis and perineal and rectal pain syndromes.
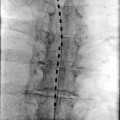
The DRG is another potential target for neuromodulation (Fig. 1.2). The DRG is reliably located intraspinally between the pedicles of the neural foramen. This structure has been targeted with radiofrequency energy to treat radicular neuropathic pain. It may be that a reversible treatment like neuromodulation is preferable to a destructive technique like radiofrequency. In the lumbosacral region, a retrograde approach is generally used and the electrode into the neural foramen. The difference between this target and SNRS is that the electrode is advanced farther into the foramen with this procedure, isolating a single dermatome and acting on the sensory cell bodies of the DRG. Although it may be more effective for this dermatome, it has less breadth of coverage than SNRS which can capture several nerve roots.
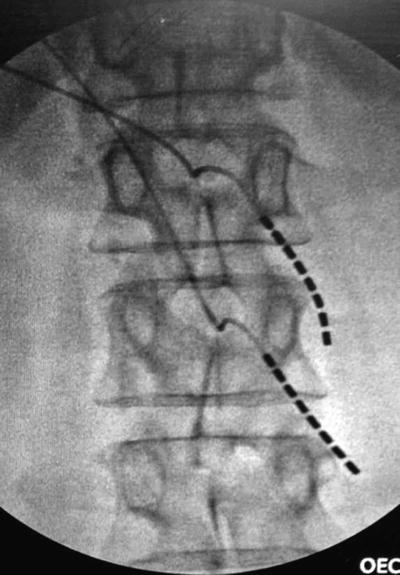

Fig. 1.2
DRG stimulation for postherpetic neuralgia. This patient had worsened symptoms with dorsal column neuromodulation. This arrangement of two leads stimulating the sensory neuronal perikarya at L1 and L2 provided 100 % relief with subthreshold stimulation (amplitude 0.5–0.8 mA with pulse width 120) (Photo courtesy of Dr. Christopher Vije, MD)
Peripheral Nervous System
Peripheral nerves may be individually stimulated or an electrical field generated through an electrode array placed subcutaneously [41]. In peripheral nerve stimulation, an attempt should be made to direct the electrode along the trajectory of the target nerve. Common peripheral nerves treated with neuromodulation include ilioinguinal nerves for post-herniorrhaphy pain, greater and lesser occipital nerves for occipital neuralgia, intercostal nerves for rib pain, and lower extremity nerves (saphenous, peroneal, tibial, sural) for foot pain. One may also use a combination of intraspinal and peripheral stimulation to treat, for instance, back and leg pain [42]. The lead is placed in proximity to the nerve rather than in contact to the nerve with most cases. In some cases, often because of lead migration or failure to capture with appropriate coverage, a paddle-type electrode is recommended for implantation.
In some cases it is not possible to isolate a single nerve branch that is responsible for the pain problem. Implantation of dual electrodes with appropriate spacing will generate a peripheral field that captures the pain problem. There is evidence that the two leads can cross talk to complete an electrical circuit and are thus creating a true field and not functioning independently [43]. This has been performed for a variety of conditions including lower back pain and abdominal pain [44].
Intrathecal Drug Delivery
Carefully selected patients may benefit from the implantation of an intrathecal drug-delivery system, typically an opioid with or without an adjunctive medication. These patients have failed more conservative options and have poor benefit and/or unacceptable side effects from oral medications. It is considered a good option for some patients with cancer pain who require large doses of opioid and suffer from severe constipation or sedation.
Typically, the catheter enters the intrathecal space at the lumbar level and is tunneled to a programmable, refillable pump usually in the lower quadrant of the abdomen. The catheter tip should be advanced to the optimal spinal level for the worst pain. For instance, back pain should have the catheter delivering medication at T10 or to the upper cervical spine for head and neck pain. These pumps are not without risk; beyond the immediate surgical risks of hematoma, cord injury, and infection, granulomas may form over time and require surgical intervention.
Pumps are typically filled with morphine, but the interventionalist may use other opioids such as hydromorphone or fentanyl. Common adjuncts to the opioid are clonidine or a local anesthetic such as bupivacaine. Clonidine takes advantage of the endogenous alpha-2 receptor mechanisms of spinal analgesia but can be complicated by hypotension or sedation. Intrathecal bupivacaine can cause numbness, edema, incoordination, or urinary retention; in other cases, it is difficult to deliver a clinically significant amount of bupivacaine, given that bupivacaine cannot be concentrated beyond 0.75 % and the low volumes required for intrathecal infusion.
Intrathecal opioid pumps produce their analgesia through an action on the mu-opioid receptors which are equally distributed on presynaptic fibers and postsynaptic neuronal cell bodies in the dorsal horn. Over time, typically several years, significant tolerance can develop. The loss of GABAergic inhibitory interneurons in the dorsal horn secondary to direct morphine neurotoxicity is thought to be one mechanism of tolerance development [45]. This is one factor that has prompted the development of alternative agents, such as ziconotide [46]. Ziconotide acts as a N-type voltage-dependent calcium channel. It blocks the release of glutamate and pro-nociceptive peptides in the dorsal horn. It has a narrow therapeutic window, with significant side effects of sedation, hallucinations, and dizziness. It has the benefit of no apparent development of tolerance or dependence, however. It is currently used for severe neuropathic pain refractory to other therapies.
Future Directions
Functional Imaging of Pain
PET and fMRI have disclosed activations in certain brain areas with chronic pain, including the anterior cingulate and insular gyri and the somatosensory cortices and thalamus. In some studies, standard MRI has shown reductions in gray matter volumes as a consequence not cause of the chronic pain of such common conditions as irritable bowel syndrome and chronic back pain [47–49]. In the case of chronic low back pain, effective treatment in one study showed reversal of the gray matter changes [50]. Should reliable protocols for common conditions such as back pain be developed, imaging studies could become outcome measures for interventional therapies.
Neuropathic Pain
Technological improvements in neuromodulation will continue to enhance their efficacy and improve our ability to treat certain pain states. Chief among these improvements, already on the horizon, is the development of small, self-contained stimulator devices that may be placed directly next to the nerve root or peripheral nerve, obviating the need for tunneling electrode leads to a battery pack. This will improve accessibility of the DRG and peripheral nerve targets, in particular, to neurostimulation.
The field of neuromodulation, like most other in medicine, would benefit from cohort studies of these different approaches used in different neuropathic pain states. Although the utility of dorsal column neuromodulation for failed back surgery syndrome/lumbosacral nerve root injury syndrome is well-established [37, 51, 52], to date, only anecdotal reports exist for the utility of this and alternate neuromodulatory strategies for other chronic neuropathic conditions. For instance, one case of central pain from multiple sclerosis was successfully treated by INRS [53]. Positive results from SNRS have been reported for lumbosacral nerve injury syndrome, ilioinguinal neuralgia, vulvodynia, interstitial cystitis, neuropathic extremity pain, and pelvic and rectal pain [54–59]. Subcutaneous peripheral nerve or field stimulation has been tried for neuropathic head and neck pain, occipital neuralgia, inguinal neuralgia, and chronic pelvic or abdominal pain [60–65].
“Sensory profiles” for neuropathic pain could enhance the study of alternate strategies and indications for neuromodulation. The concept proposes that a particular pattern of sensory description corresponds to a specific physiological change, even among patients with the same disease process. Thus, allodynia may represent a greater GABAergic inhibitory deficit in the dorsal horn; numbness, a significantly greater degree of deafferentation; spontaneous pain, a greater degree of Aδ fiber activity; and so on. Physiology in turn determines the efficacy of neuromodulation. These “sensory profiles” could then be used to stratify patients within a population to address the issue of nonresponders.
A case in point is postherpetic neuralgia, where dorsal column neuromodulation and peripheral field stimulation have both been reported effective in several patients [66, 67], yet is a condition where neurostimulation is not generally regarded as a useful modality [68, 69]. A descriptive study of 2,100 patients with either PHN or DPN demonstrated differences in hyperalgesia and allodynia between the two populations [70]; five patterns of sensory symptom description were detected within these two populations although differing in frequency within each. Distinct neuropathic signs and symptoms in PHN (i.e., paroxysmal vs. continuous pain) are generated by different patterns of abnormality among primary afferent neurons (Aβ- vs. Aδ- and C fibers). This type of research where clinical description is matched to underlying physiology may point the way forward, in identifying subtypes of neuropathic pain responsive (or not responsive) to different approaches to neuromodulation. This could greatly reduce the number of unnecessary trials and failed implants (see Fig. 1.2).
Visceral and Autonomic Systems
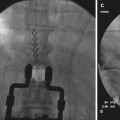
There exists a great deal of information on the utility of dorsal column neuromodulation for chronic stable angina and non-reconstructable lower extremity ischemia (Fig. 1.3) [38, 71, 72]. These indications are more widely used in Europe; practitioners in the USA have not managed to partner with vascular surgery, cardiology, and primary care in such a way as to be able to provide this treatment modality to the appropriate patients effectively. There is also burgeoning interest in the use of cervical spinal cord stimulation to increase cerebral perfusion in low-flow states, including enhancing chemotherapy delivery to brain tumors and improving cerebral oxygenation in patients poststroke [73, 74].
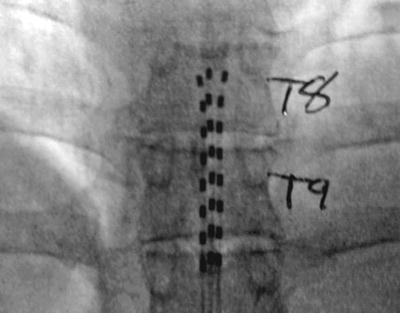

Fig. 1.3
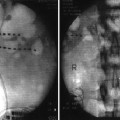
Dorsal column tripole configuration. Using an Octrode lead on either side of a third Octrode allows one to “guard” the midline lead with positive charge. This drives the stimulation deeper into the dorsal columns and can prevent limb and thoracic dermatomal paresthesias. This arrangement has been used for chronic pancreatitis and axial low back pain
For chronic visceral pain such as pancreatitis, a guarded tripolar lead array is frequently used to drive stimulation deeper into the dorsal columns. Presumably, this allows activation of fibers in the midline visceral pain pathway which engages inhibitory mechanisms in deeper laminae VII and X where visceroceptive neurons reside. This existence of this pathway has led some to promote the efficacy of T10 midline punctuate myelotomy for intractable cancer-related pelvic pain [75], which may be considered by the surgical interventionalist in their palliative care population.
Intrathecal Drug Delivery
Currently available technology would benefit greatly from novel analgesics to deliver intraspinally. Gabapentin, a well-established drug for the treatment of neuropathic pain, is one such candidate for intrathecal administration [76, 77]. Alternatively, it may be that adjuncts to morphine, such as baclofen, enhance its efficacy and reduce tolerance development [78]. An intriguing possibility in this regard would be medications to inhibit proinflammatory mediators in the spinal cord. Cytokine and chemokine activation appears not only to drive neuropathic pain itself, but to specifically reduce analgesia and promote tolerance development associated with opioids [79, 80].
Summary
Pain is a neurological condition that affects every level of the nervous system. The most reasonable target for therapy remains the peripheral nerve and spinal dorsal horn, as first proposed by the gate theory; at more rostral levels, pain-related activity is distributed among a “pain matrix” whose complexity makes it difficult to act upon. Imaging of this pain matrix, however, may become an objective surrogate marker for studies of pain and its treatment. Studies of neuromodulation for a variety of neuropathic pain states will benefit from correlation with clinical characteristics and physiology to reduce the number of failed implants. New medications, either alone or adjunctive to intrathecal opioids, will make infusion pumps a more attractive modality for pain control.
References
2.
3.
Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci. 1993;13(6):2273–86.PubMed
4.
5.
6.
7.