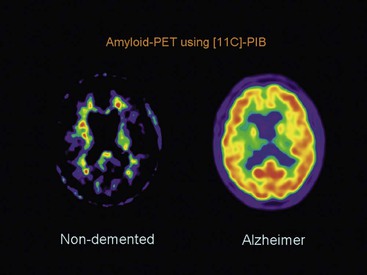
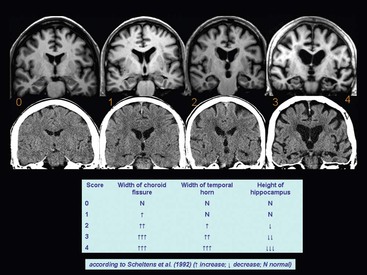
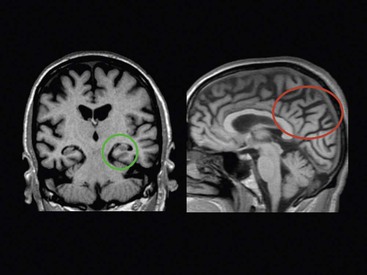
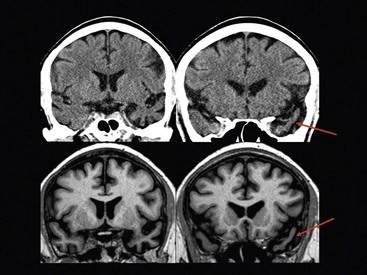
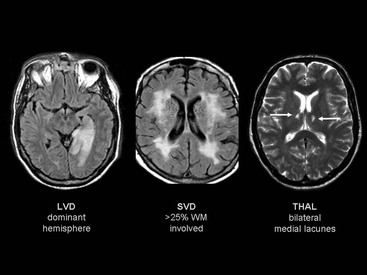
Beatriz Gomez Anson, Frederik Barkhof With the rising age of the population, both normal ageing phenomena in the brain and neurodegenerative disorders become more prevalent. While exceptionally the brain may not be affected by age (successful ageing), the more typical or unusual ageing involves general involutionary alterations, which may mimic or herald neurodegenerative disease. Clinically, mild memory loss and reduced processing speed are considered normal for age, and can only be objectively established with extensive neuropsychological testing. Dementia is a clinical syndrome that is defined as an acquired condition involving multiple cognitive impairments that are sufficient to interfere with activities of daily living, and often is progressive. Alzheimer’s disease (AD) is the most common cause of dementia and often presents with memory impairment, but in other diseases like frontotemporal dementia (FTD), behavioural or language problems may prevail. Diagnosis is critically dependent on careful history taking from patient and informant, followed by clinical and cognitive examination supported by ancillary investigations, of which neuroimaging is one of the most important. The a priori chance of a particular disease being present is dependent on age. In younger patients, more rare disease may occur and FTD is relatively common, although AD is still the most prevalent disorder. In the older patients, AD, dementia with Lewy bodies (DLB) and vascular disease are the most common. Mixed AD and vascular disease is the most frequent pathology in the elderly (>85 years). Genetic causes of dementia are important in FTD, but only explain 1–2% of (presenile) AD cases. Neoplasms rarely present with cognitive decline. Ancillary investigations in the work-up of suspected dementia are quite important, since clinical diagnosis has a relatively low accuracy compared to histopathology. Cerebrospinal fluid (CSF) analysis plays an important role by examining levels of β-amyloid and (phosphorylated) tau. Neuroimaging is the most important ancillary investigation in the work-up of suspected dementia, and should be combined with clinical, neuropsychological and laboratory data in a multidisciplinary conference to enhance diagnostic accuracy. Despite the absence of definitive treatment for most disorders, establishing a correct nosological disorder is important in terms of counselling and planning, and identifying relevant (vascular) comorbidity. Normal ageing may be subdivided into successful ageing (without any discernible changes) and the more commonly observed typical (usual) ageing. Typical ageing includes a variety of changes, including overall brain shrinkage, but also local alterations, such as white matter changes. Many of these ‘normal’ ageing phenomena have been linked to risk factors (e.g. vascular) and although cognitive function may seem intact, subtle abnormalities may be detected on detailed neuropsychological testing. Such relationships are often only discernible on a group level, and predictions in individual subjects are difficult to provide. Table 65-1 lists the alterations observable in typical/usual ageing. The focus of imaging in suspected dementia has shifted from an exclusionary to an inclusionary approach.1 Exclusion of a (surgically) treatable cause of dementia (e.g. tumour or subdural haematoma) can be ascertained by using computed tomography (CT), but demonstration of positive disease markers (e.g. hippocampal atrophy for AD) becomes increasingly more relevant and magnetic resonance imaging (MRI) adds positive predictive value to the diagnosis in dementia. Catheter angiography (DSA) is hardly ever indicated, except perhaps for suspicion of vasculitis. While MRI is the modality of choice for investigating dementia, multislice CT offers a reasonable alternative, with coronally reformatted images enabling examination of the medial temporal lobe. However, CT is still clearly inferior to MRI, for example, in subjects suspected of having some rare disorders causing dementia, such as encephalitis or Creutzfeldt–Jakob disease (CJD). When structural imaging is equivocal or does not lead to the diagnosis, functional imaging may add diagnostic value.2 Second-line investigations include metabolic information obtained by using single-photon emission computed tomography (SPECT) or positron emission tomography (PET), or physiological information obtained by using diffusion or perfusion MRI. For example, in the early stages of frontotemporal lobar degeneration (FTLD) without discernible atrophy, fluorodeoxyglucose PET (FDG-PET) or hexamethylpropyleneamine oxime SPECT (HMPAO-SPECT) may already demonstrate decreased metabolism or hypoperfusion. Molecular imaging provides even more early and specific information, e.g. amyloid-PET in Alzheimer’s disease (Fig. 65-1) and dopaminergic tracers in Lewy-body dementia. Sructured reporting is essential in dementia3 and should consider: The disease is named after Aloïs Alzheimer, who first described senile plaques and neurofibrillary tangles in a 51-year-old woman in 1906. Although the aetiology of AD is uncertain, the amyloid cascade predicts that abnormal aggregation of amyloid leads to impaired nerve function, formation of extracellular amyloid (neuritic) plaques and subsequent formation of intracellular neurofibrillary tangles, leading to neuronal loss and atrophy. The process usually starts in the medial temporal lobe (entorhinal cortex and hippocampus) or the posterior cingulate, and then spreads to the tempoparietal cortex. In <1% of cases, a mutation in genes encoding for amyloid-processing enzymes is found; however, most cases are sporadic, with APOE4 genotype increasing the risk of AD moderately. Age and cardiovascular risk factors are important predictors as well. The clinical manifestations of AD are episodic memory impairment, but in younger patients visuospatial disturbances may prevail, and even language problems can be seen. While a clinically probable AD diagnosis requires interference in at least two separate domains, biomarkers such as CSF amyloid, PET and MRI can be useful in the prodromal stage of mild cognitive impairment (MCI) by providing evidence of amyloid pathology and subsequent neurodegeneration. The conversion rate from MCI to AD is 10–15% per year and this risk increases markedly when MRI shows atrophy suggestive of AD, e.g. hippocampal atrophy. MRI and CT are both useful for excluding surgically treatable disorders and demonstrate focal atrophy suggestive of AD.4 Coronal images perpendicular to the long axis of the temporal horn should be used to determine the amount of hippocampal and medial temporal lobe atrophy (MTA). Since volumetric analysis of the hippocampus is quite time-consuming, MTA can best be analysed using a visual rating scale depicted in Fig. 65-2. A score of 2 is considered abnormal under the age of 75, and a score of 3 above that age. Strong asymmetry between left and right side should trigger a suspicion of FTD, which can be recognised by concomitant atrophy of the temporal poles and frontal lobes. In younger subjects with AD, the hippocampus may be spared, with pathology dominating in the precuneus (including posterior cingulate) and parietal cortex (Fig. 65-3). Vascular changes and AD are common and therefore coexisting pathologies in the elderly. The combination of infarcts and Alzheimer pathology is the strongest predictor of dementia in population-based autopsy studies. Atherosclerosis may lead to ischaemic brain damage and probably also accelerates the formation of Alzheimer pathology. In individual patients, it may be difficult to pinpoint their respective relevance, but vascular alterations, especially white matter lesions on MRI, provide an independent target for treatment. Treatment of vascular risk factors may not only prevent further vascular pathology but also benefit progress of AD. The term FTLD describes a group of disorders with tau pathology presenting with language and behavioural symptoms. FTLD is the third most common degenerative cause of dementia after AD and dementia with Lewy bodies, accounting for about 5–10% of all cases of dementia and, in younger patients, it is second in frequency after AD. Arnold Pick first described patients with focal atrophy of the frontal and temporal lobes in 1892, including patients with both personality change and language impairment. The Lund-Manchester criteria recognise three main subtypes of FTLD: Less commonly, patients with right-sided FTLD present with difficulty recognising faces. It should be noted that language and behavioural disturbances can also be seen in (atypical) AD cases and corticobasal degenration (CBD) and progressive supranuclear palsy (PSP). In contrast to AD, a significant proportion of patients with FTLD have an autosomal dominant family history, sometimes linked to chromosome 17, which can be due to mutations in the microtubule-associated protein tau (MAPT) or progranulin. Other mutations can be found as well, especially in patients with increasingly recognised overlap syndromes with motor neuron disease and amyotrophic lateral sclerosis (ALS). The hallmark imaging finding in FTLD is atrophy of the temporal and frontal lobes, often (initially) asymmetrical (Fig. 65-4). The patients presentating with the language variant SD often have marked left temporal lobe atrophy (temporal pole more than hippocampus), but in patients with bvFTD, atrophy of the frontal lobes can be mild at the time of presentation. In particular, in these patients, FDG-PET and perfusion MRI can be useful detect functional changes, although care should be taken to exclude depression, which may be similar clinically and on imaging. Vascular dementia (VaD) is the second most common type of dementia after AD, especially in the elderly (where they often coexist and even reinforce one another). The term VaD implies the existence of dementia; however, it is often hard to prove dementia is indeed secondary to cerebrovascular disease (only). Furthermore, VaD is a heterogeneous entity and comprises various conditions due to small- or large-vessel involvement: • Diffuse confluent age-related white matter changes (ARWMC): • also referred to as subcortical arteriosclerotic encephalopathy (SAE). • Multilacunar state (‘état lacunaire’). • Multiple (territorial) infarcts. • Strategic cortical–subcortical or borderzone infarcts. • Cortical laminar necrosis (granular cortical atrophy). • Delayed post-ischaemic demyelination. Clearly, not every vascular pathological finding seen on brain MRI or CT is sufficient to associate with occurrence of dementia in a given patient.5 The NINDS-AIREN (National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l’Enseignement en Neurosciences) criteria are the most strict ones for VaD and detail various causes of small- and large-vessel pathology that are likely to cause dementia (Fig. 65-5), which we will discuss in the following sections. Dementia may result from multiple or single cortical–subcortical or subcortical (e.g. borderzone) cerebrovascular lesions (infarcts) involving strategic regions of the brain, such as the hippocampus, paramedian thalamus and the thalamocortical networks, especially if they occur in the dominant hemisphere. Also referred to as leukoaraïosis or subcortical arteriosclerotic encephalopathy, extensive small-vessel disease due to microangiopathy is the most common form of VaD. Most of these cases are idiopathic or sporadic; i.e. there is no proven or specific/genetic cause that can be identified, although many patients will have a history of cardiovascular risk factors. Within the group of small-vessel VaD, the following subtypes exist: • extensive white matter lesions—confluent hyperintensities involving >25% of WM; • multiple lacunes—at least two lacunes in basal ganglia and centrum semiovale each; and • bilateral thalamic lesions—small infarcts in both medial thalami.
Neurodegenerative Diseases and Epilepsy
Ageing and Dementia—Introduction and Clinical
Normal Ageing Phenomena in the Brain
Dementia—Imaging Approach
Indications for Imaging
Protocol for CT and MRI
Alzheimer’s Disease and Other Primary Neurodegenerative Dementias
Alheimer’s Disease
Frontotemporal Lobar Degeneration
Vascular Dementia
Large-Vessel VaD
Small-Vessel VaD
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Neurodegenerative Diseases and Epilepsy
Chapter 65