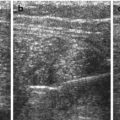
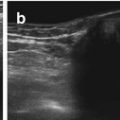
Fig. 3.1
Trapezoid view using high-resolution linear transducer for transfontanellar brain US. Normal neonatal brain US in a coronal section through foramen of Monro (a) and sagittal section in midline (b)
Transtemporal Access:
Transtemporal, transmastoid or other accesses such as occipital fontanel used in neonates for detailed assessment of certain brain areas.
In older children only transtemporal access with sector (vector) transducers (frequency 7–1 MHz) is applicable.
3.1.2 Indications for Brain US
Indications in Neonates and Young Infants
Typical standard indications:
Asphyxia and birth trauma, prematurity (screening, i.e. 3rd and 7th day).
Macro- or hydrocephalus and (fetally) suspected cerebral malformations.
Suspicion of brain haemorrhage.
Clinical neurologic symptoms.
Disease potentially associated with cerebral manifestations (e.g. septicaemia with brain abscess, tuberous sclerosis and syndromic disease), meningeal empyema, meningoencephalitis, brain abscess, etc.
Some centres perform screening neonatal brain US.
Indications in Older Children:
Hydrocephalus, also after shunting – using transtemporal access.
Suspected cerebral perfusion alteration, such as with cardiac malformations, after hypoxia or drowning, sickle cell disease, etc.
Cerebral perfusion can be monitored at bedside in ICU for optimising ventilation (“individual optimal PCO2”).
Assessment of (post-)haemorrhagic or tumorous midline shift, large vascular malformations or brain death.
3.1.3 How to Investigate
Positioning
Child in stable position – supine or lateral decubitus.
NOTE: Avoid pressure of transducer on fontanel.
Transfontanellar Access:
Assessment of entire visible brain in serial coronal and (para-)sagittal sections (Fig. 3.2).
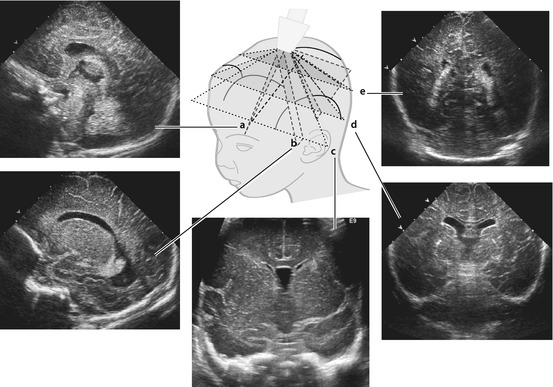
Fig. 3.2
Transfontanellar US – typical sections + respective images that should be documented. Standard sections in scheme with corresponding respective images of normal US appearance. a sagittal midline, b parasagittal, c anterior coronal, d median coronal and e occipital coronal
Documentation of anterior, middle and posterior fossa as well as midline structures and lateral ventricles. Important features that need to be documented: corpus callosum, ventricles including foramina of Monro with measurements if enlarged, temporal and occipital horn of lateral ventricles, basal ganglia, peripheral parenchyma and interhemispheric fissure.
In case of trauma or palpable bumps – assess calvarium using high-resolution linear transducers.
For special queries – add transmastoid, transtemporal or transoccipital access (e.g. visualisation of aqueduct, posterior fossa structures and cerebellum particularly in preterms, medulla oblongata), basal vessels.
CDS: basal vessels, compare flow between left and right hemisphere.
NOTE: Use same routine for every investigation in order not to miss anything – systematic consistent approach.
Transtemporal, Transcranial, Mastoid Access, etc.:
Every less ossified part of the calvarium can be used, also osseous defects such as after surgery.
Axial and transverse sections acquired by rotating transducer.
Coronal and oblique sections obtainable as needed.
Morphometry and Documentation:
Standardised image orientation:
Left side of US image = frontal (sagittal/axial view) and right side (coronal view).
Always use proper labelling or pictograms.
Standardised measurement of ventricular diameter and size, particularly if enlarged (Fig. 3.3).
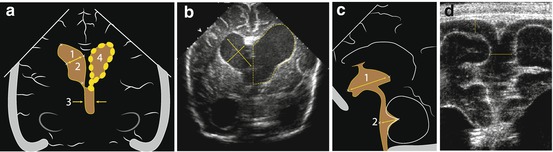
Fig. 3.3
Measurements on brain US. (a) Standard measurements of lateral (1, 2) and third (3) ventricle diameter at level of the foramen of Monro in coronal section; sometimes area (4) and circumference used (….) – more sensitive towards subtle changes. (b) Coronal section, hydrocephalus: ventricle measurements – diameters, circumference and area. (c) Standard depth measurements of third (1) and forth (2) ventricle in sagittal midline section. (d) Coronal section, linear transducer, near filed view: extra-axial CSF size and interhemispheric fissure distance measurements (↔)
Width of extra-axial CSF space: interhemispheric fissure, subfontanellar space, fronto-temporal space (e.g. arachnoid cyst) and sylvian fissure – see also hydrocephalus.
Width of brain parenchyma (anterior, occipital, temporopolar) (Fig. 3.4).
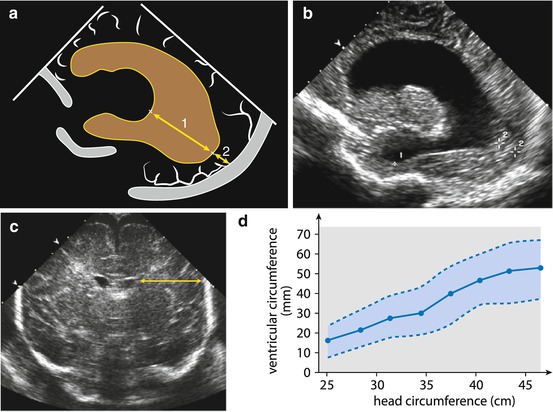
Fig. 3.4
Additional measurements. Measurement of parenchymal width (↔), particularly important in hydrocephalus. (a) Parasagittal scheme: measurement of ventricular distance (1) and occipital parenchymal width (2). (b) US image in parasagittal section: hydrocephalus with severely narrowed frontal and occipital parenchyma (+1, +2). (c) Coronal section: parenchyma (↔) to ventricle ratio can be calculated, useful for follow-up incorporating physiological growth with stable relations in spite of increasing values. (d) Normal values for ventricular size (mm, circumference of lateral ventricle at level of foramen of Monro) related to head circumference (cm)
Size of the cerebellum (Fig. 3.5, Table 3.1).
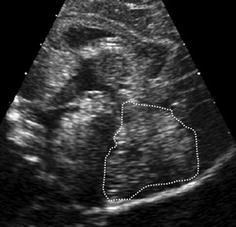
Fig. 3.5
Planimetric measurement of cerebellar size. Cerebellum (dotted line) outlined for planimetry – age adapted normal values see Table 3.1
Table 3.1
Cerebellar size in preterm and term neonates
Gestational age (week)
Body weight (g)
Cerebellar area (cm2)
26
600
0.75
28
820
1.30
30
1,330
1.95
32
1,580
2.43
34
1,900
2.66
36
2,220
3.18
38
2,840
3.56
40
3,100
3.98
Minimum documentation includes the following:
Three to four coronal views (anterior, foramen of Monro, posterior horn, periventricular posterior parenchyma).
Three to five sagittal views (midline, left and right parasagittal through lateral ventricles, periventricular parenchyma on both sides).
Every abnormality documented in two orthogonal planes, reproducible measurements helpful.
Additional views helpful: brain surface with linear transducer, mastoid view for posterior fossa, and CDS images with respective duplex trace (ICA, ACA, MCA, circle of Willis, BA, major veins).
3.2 Normal Findings
3.2.1 Transfontanellar Access
Ventricular System:
Slim lateral ventricles with symmetric configuration, <10 mm diameter at level of foramen of Monro.
Normal CSF: without any echoes.
Choroid plexus: echogenic structure partially outlining ventricle, in posterior parts floating within CSF.
Ventricular system used as landmark for most parts of brain.
Other Physiologic Cavities to Visualise:
Various cisterns.
Various embryologic remnants such as cavum septi pellucidi, cavum vergae, cavum velum interpositum.
Extra-axial CSF space (interhemispheric fissure).
Brain Parenchyma:
More or less homogeneous, of medium echogenicity, slight difference between darker cortex and more echogenic white matter, identified echogenic sulci (may be inverted with immaturity, oedema etc.).
Basal ganglia and internal capsule identified by different echogenicity with high-resolution transducers.
Major vessels appear as echogenic pulsating tubular or nodular structures.
3.2.2 Alternate Access Findings
Image appearance varies with access; usually important basal and central structures can easily differentiated:
Lateral ventricles, foramen of Monro, third and fourth ventricle, connecting aqueduct of Sylvius, crura cerebri, major parts of basal ganglia, tentorium, parts of cerebellum, basal vessels and contralateral extra-axial CSF spaces.
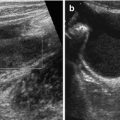
Mastoid access: occipital horn with choroid plexus, adjacent parenchyma (Fig. 3.6) and cerebellum.
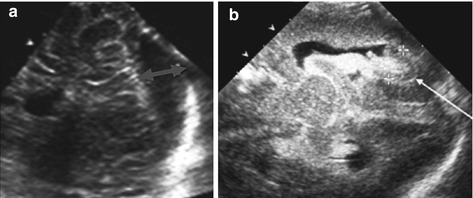
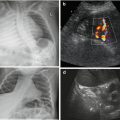
Fig. 3.6
Mastoid access with sector transducer. (a) Posterior fossa, axial view; note large cistern (↔). (b) Axial view at posterior horn (→) of lateral ventricle with haemorrhage (+ +) and respective plexus
3.2.3 Colour Doppler Sonography (CDS)
Transfontanellar:
Anterior cerebral artery (ACA) and its branches (particularly the pericallosal) and basilar artery (BA) – best seen in sagittal sections (Fig. 3.7a, c).
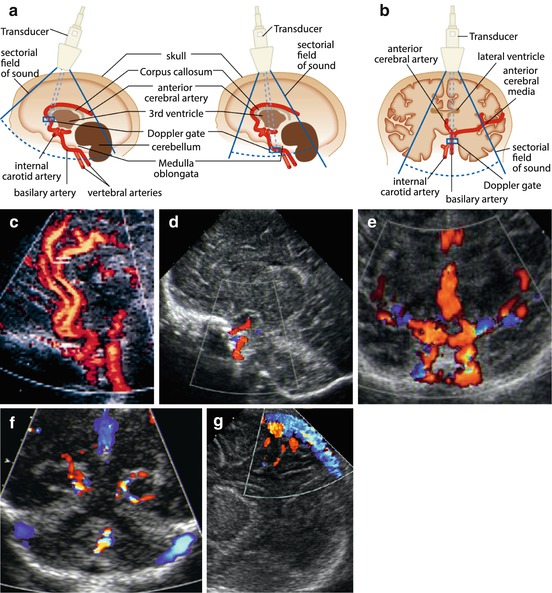
Fig. 3.7
Major cerebral vasculature on aCDS. (a) Schematic drawing how to access the ACA/pericallosal artery and the basilary artery (BA) in a sagittal section. (b) Schematic drawing how to access in a coronal section. (c) Sagittal midline section, aCDS: BA, ICA, ACA and branches seen, duplex sample positioned in pericallosal artery. (d) Parasagittal section CDS depicts the ICA when entering into intracranial portion. (e) Coronal CDS demonstrates both ICA as well as the branching into MCA and ACA. (f) CDS, coronal section tilted occipitally: major veins/venous sinus of posterior fossa depicted (displayed in blue) and branches of PCA. (g) CDS in sagittal section to evaluate midline veins, particularly the superior sagittal sinus. Abbreviations:(a) CDS (amplitude) coded colour Doppler sonography, BA basilar artery, ICA internal carotid artery, ACA anterior cerebral artery, MCA middle cerebral artery and PCA posterior cerebral artery
Circle of Willis with its major feeding arteries and draining veins – best seen in coronal section (Fig. 3.7b, e).
Internal carotid artery (ICA) – in parasagittal or coronal section (Fig. 3.7b, d, e).
NOTE: In patients who need cannulisation of CCA (e.g. for ECMO) or after CCA injury, visualisation of anterior communicating artery is particularly important – if seen, it indicates sufficient collateralisation of affected ACA area. Liberally use CDS, complemented by spectral analysis in relevant vessels.
Transtemporal
Most vessels of circle of Willis are visible (Fig. 3.8a), particularly:
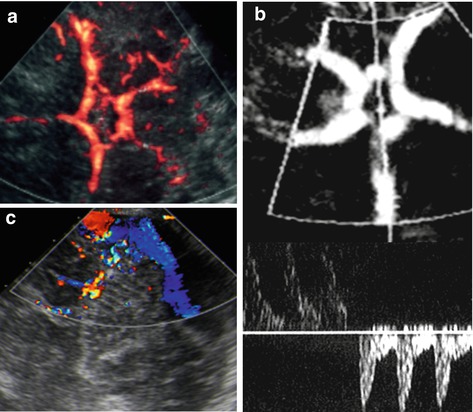

Fig. 3.8
aCDS – transcranial/transtemporal access (TCI). (a) Major arteries of circle of Willis depicted by aCDS. (b) The TCI view used to place sample gate for spectral analysis with a normal bidirectional arterial flow pattern form left and right MCA in the lower part of the image. (c) CDS on TCI/posterior fossa: veins/sinus of posterior fossa can be assessed
Middle cerebral artery (MCA), proximal part of ACA (A1-segment).
Proximal part of posterior cerebral artery (PCA), posterior and anterior communicating arteries.
Depiction of (proximal) ICA, distal ACA and BA difficult or impossible.
This view used for transcranial flow evaluation (Fig. 3.8b).
Venous System
Most veins visible and better seen from transfontanellar access (Fig. 3.7f, g), for example:
Superior sagittal sinus, inferior sagittal sinus, sinus confluence (torcular herophili), vein of Galen and sigmoid and transverse sinus.
Large sinus of posterior fossa – additionally use mastoid or transtemporal approach (Fig. 3.8c).
NOTE: For assessment of large veins, transducers with sufficient penetration at lower frequencies may be necessary and flow seetings have to be adapted to low flow velocities.
Measurements
Arterial flow velocities as well as RI vary with age, but also with respiration, PCO2, intrathoracic pressure (crying, ventilation, etc.), cardiac function, heart rate, etc. Cerebral autoregulation only intact in mature healthy brain – then peripheral vessels show less dependent flow variations. General rule:
Flow velocities increase with age, RI decrease with age (in childhood).
Respiratory variation typical of venous flow may persist.
Preterms: flow velocities 20–40 cm/s, RI = 80–90 %.
Neonates: flow velocities 40–50 cm/s, RI = 75–80 % (see Table 3.2).
Table 3.2
Brain: Normal values for flow velocities (cm/s) and Doppler indices in ACA, ICA and BA in newborns
ACA | BA | ICA | |
|---|---|---|---|
Vs | 42 + 15 | 41 + 16 | 50 + 15 |
Ves | 19 + 8 | 18 + 7 | 19 + 7 |
Ved | 11 + 5 | 11 + 4 | 11 + 4 |
TAV | 13 + 5 | 12 + 5 | 14 + 4 |
TAMX | 21 + 9 | 19 + 7 | 22 + 6 |
RI | 0.73 + 0.08 | 0.72 + 0.09 | 0.77 + 0.08 |
PI | 2.7 + 0.9 | 2.7 + 0.7 | 3.0 + 0.8 |
Older neonates and infants: assessed by transtemporal access. Flow velocities increase; RI decreases to 60–70 %, eventually reach adult values by end of first year. Physiologic decrease of arterial diastolic velocity with consequent elevated RI values in patients with persistent ductus arteriosus (PDA) or anaemia (Table 3.3).
Table 3.3
Brain: Normal values for flow velocities and Doppler indices on TCI-Doppler investigations
Flow velocity | Artery | Age | ||||
|---|---|---|---|---|---|---|
1–2.9 years | 3–5.9 years | 6–9.9 years | 10–17.9 J years | Adults | ||
Vs (cm/s) | MCA | 124 ± 10 | 147 ± 17 | 143 ± 13 | 129 ± 17 | 110 ± 28 |
ACA | 81 ± 19 | 104 ± 22 | 100 ± 20 | 92 ± 19 | 79 ± 21 | |
ICA | 118 ± 24 | 144 ± 19 | 140 ± 14 | 125 ± 18 | ||
PCA1 | 67 ± 18 | 84 ± 20 | 82 ± 11 | 75 ± 16 | 71 ± 16 | |
ICA (siphon) | 114 ± 21 | 138 ± 14 | 132 ± 17 | 120 ± 21 | ||
BA | 71 ± 6 | 88 ± 9 | 85 ± 17 | 68 ± 11 | ||
Ved (cm/s) | MCA | 65 ± 11 | 65 ± 9 | 72 ± 9 | 60 ± 8 | 49 ± 14 |
ACA | 40 ± 11 | 48 ± 9 | 51 ± 10 | 46 ± 11 | 35 ± 11 | |
ICA | 58 ± 5 | 66 ± 8 | 68 ± 10 | 59 ± 9 | ||
PCA1 | 36 ± 13 | 40 ± 12 | 42 ± 7 | 39 ± 8 | 33 ± 14 | |
ICA (siphon) | 55 ± 12 | 62 ± 8 | 67 ± 11 | 58 ± 12 | ||
BA | 35 ± 6 | 41 ± 5 | 44 ± 8 | 36 ± 7 | ||
RI | MCA | 0.47 ± 0.05 | 0.55 ± 0.05 | 0.50 ± 0.05 | 0.53 ± 0.05 | 0.56 ± 0.06 |
ACA | 0.55 ± 0.05 | 0.57 ± 0.05 | 0.57 ± 0.05 | 0.58 ± 0.05 | 0.56 ± 0.07 | |
ICA | 0.52 ± 0.05 | 0.60 ± 0.05 | 0.55 ± 0.05 | 0.58 ± 0.05 | ||
PCA1 | 0.55 ± 0.05 | 0.58 ± 0.05 | 0.55 ± 0.05 | 0.55 ± 0.05 | 0.54 ± 0.06 | |
ICA (siphon) | 0.57 ± 0.05 | 0.63 ± 0.05 | 0.55 ± 0.05 | 0.58 ± 0.05 | ||
BA | 0.55 ± 0.05 | 0.60 ± 0.05 | 0.55 ± 0.05 | 0.57 ± 0.05 | ||
Functional Investigations
Changes in flow spectrum, velocities and indices are observed with head positioning, particularly important for depicting infants at risk for sudden infant death syndrome.
Physiologic variations in flow velocities and RI indices with change of PCO2 – used to monitor cerebral perfusion and optimise respirator settings (e.g. children under hyperventilation for treatment of brain oedema after hypoxic events).
Subtle mechanical fontanellar pressure used to assess effect on brain perfusion – useful for assessment of critical intracranial pressure elevation (e.g. hydrocephalus) (Fig. 3.9).
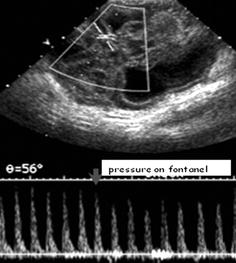

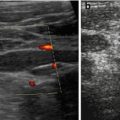
Fig. 3.9
Transtemporal DS tracing while applying fontanellar pressure. Duplex Doppler gate placed in main MCA branch on a TCI-Doppler acquisition. Note: Reduction of flow velocities when slight fontanellar compression is applied by finger tip
Differences in flow velocity and RI values between intra- and extracranial portion of ICA used to demonstrate normal, slightly or significantly elevated brain pressure (see hydrocephalus).
3.2.4 Normal Variances in Preterm Babies
3.2.4.1 Periventricular Echogenicities
Physiologically reduced differentiation between gray and white matter due to immaturity; central nonmyelinated white matter can be relatively echogenic, particularly in periventricular areas.
Periventricular white matter is frequently homogeneously and slightly increased in echogenicity (often symmetric) in severely premature and very immature neonates (i.e. “PVE” = periventricular echodensity) (Fig. 3.10).
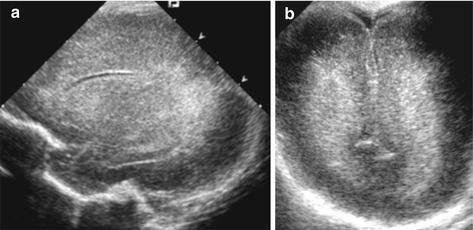

Fig. 3.10
PVE in preterm infant. Typical increased paraventricular echogenicities (“PVE”) in severely immature brain of preterm baby. (a) Parasagittal section maybe the slightly pathy appearence may indicate early PVL – only to be decided on follow-up. (b) Coronal view tilted occipitally
NOTE: Findings often difficult to differentiate from early hypoxic-oedematous changes that eventually may lead to periventricular leukomalacia (PVL).
Immature brain also exhibits reduced gyration and prominent CSF spaces (intra-/extra-axial); embryologic cavities can persist and may be remarkably prominent, with high-resolution transducers radiate coron depictable (Fig. 3.11).
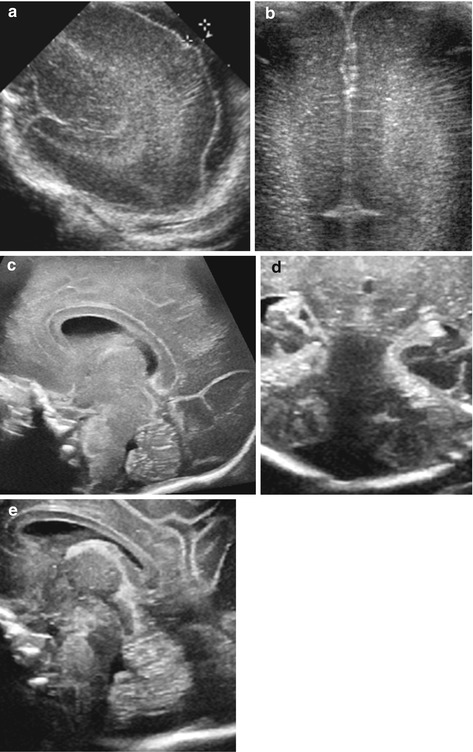

Fig. 3.11
US appearance in preterm babies – typical features and findings. (a) Parasagittal section – wide CSF space (+ +). (b) Coronal section: reduced gyration + prominent periventricular echoes with echogenic lines of the corona radiata. (c) Sagittal midline view: physiologically persisting cavum septi pellucidi et vergae. (d) Coronal midline section, posterior fossa: large cisterna magna. (e) Sagittal midline section, posterior fossa: prominent CSF space (cisterna magna)
3.2.4.2 Ventricular Asymmetry
To a certain extent, this can be seen as normal variant – does not always indicate pathology.
3.2.4.3 Ventriculomegaly
Term neonates: ventricles measuring 5–10 mm (at level of foramen of Monro) called ventriculomegaly.
In older children: ventriculomegaly without other signs of hydrocephalus measuring up to 1.5 cm axial diameter.
3.2.4.4 Cisterna Magna
Size quite variable (range 2–10 mm). Large posterior fossa CSF spaces more common in preterms (Fig. 3.11d, e).
NOTE: Sometimes differentiation of large cistern (mega cisterna magna) versus arachnoid cyst or Dandy-Walker “pseudocyst” difficult or impossible.
3.2.4.5 Vascular Variations
Many anatomic variations of brain vessels without pathologic implications are known, only some detectable by CDS.
NOTE: Many systemic or cardiac conditions may influence Doppler findings, e.g. heart malformations with shunt, cardiac insufficiency, heart rate, drugs, hypo- and hypervolaemia, anaemia, dehydration, hyperviscosity, altered respiration, PCO2 and increased intrathoracic pressure (Fig. 3.12). Furthermore, extracranial conditions or position may affect perfusion pattern in cerebral vessels (e.g. SIDS risk in infants with altered BA flow when head is extremely turned to the one or other side). Thus, not all altered spectral Doppler findings indicate brain disease or cerebral vascular problems; however, they may hint at potential perfusion disturbances with neurologic sequelae secondary to another underlying condition. Sometimes CDS is used to grade these conditions in terms of therapeutic needs (i.e. grading of PDA).
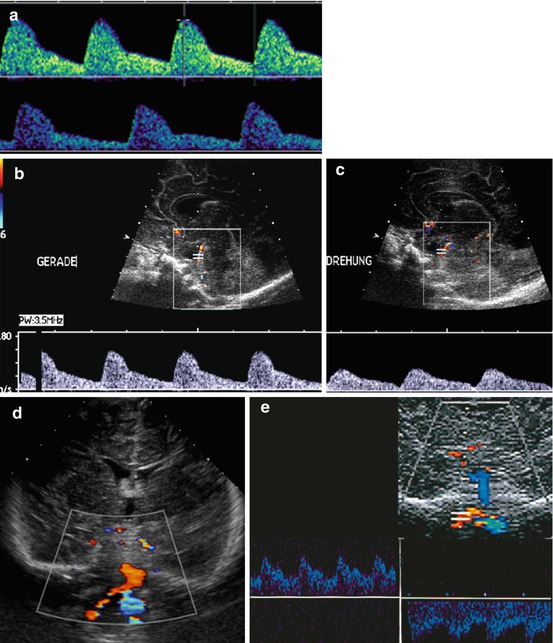

Fig. 3.12
Impact of heart rate and extracranial conditions on Doppler findings: different flow pattern, RI and V diast. (a) Diastolic velocity changes with heart rate – RI increases with bradycardia, whereas diastolic velocity decreases. (b, c) Flow alteration in BA when head is turned (“Drehung”) – normal flow in normal position (“Gerade”). (d) Retrograde flow (encoded in blue) in left vertebral artery in subclavian steal Syndrome. Duplex trace confirmation, in this example in a baby after ALTE (acute life threatening event) during extensive head turning, with consequently inverted flow direction of basilar artery (encoded in blue) (e)
3.3 Pathologic Findings
3.3.1 Neural Tube Defects
3.3.1.1 Anencephaly
Most severe malformation, diagnosed prenatally. No postnatal indication for US.
3.3.1.2 Meningomyelocele and Encephalocele
Herniation of meninges, brain parenchyma and/or spinal cord tissue through defect in osseous containment (skull, spine), parts of CSF system and ventricles can herniate as well.
US Finding
Bone defect detectable using high-resolution transducers or by transtemporal approach-from contralateral side. The anechoic CSF space protrudes into defect with variable amounts of brain herniation depending on amount and kind of defect and malformation (Fig. 3.13a).
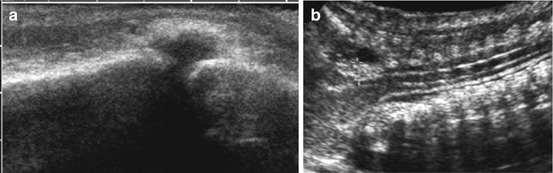

Fig. 3.13
Cerebral manifestations of neural tube defects. (a) Linear transducer on the occipital skull: small osseous defect depicted with herniation of fluid-filled meningeal pouch, consistent with a small occipital meningocele. NOTE: No brain content. (b) Curved array, sagittal view from nuchal approach, neck bent anteriorly: slightly herniated cerebellar vermis (+ +) in Arnold Chiari syndrome
3.3.1.3 Arnold Chiari Malformation
Usually associated with spinal myelo(meningo-)celes (see spine US), with subsequent disturbed relation of midline structures, particularly in posterior fossa. Typically the cerebellar vermis protrudes through foramen magnum to cervical spinal level (Fig. 3.13b).
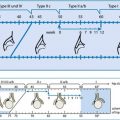
Classification and US Findings
Depending on nomenclature: grade I to grade III, trying to categorise minimal findings:
Type I: no dysraphic malformation and only slight caudalisation of lower parts of cerebellum to foramen magnum.
Type II: spinal dysraphism often combined with CSF circulation problems causing hydrocephalus.
Type III: most severe form – occipito-cervical encephalocele.
Associated malformations: agenesis of septum pellucidum, pronounced massa intermedia, atypical thickened choroid plexus of lateral ventricle, hypodysplasia of cerebellum and agenesis or hypoplasia of corpus callosum.
3.3.1.4 Dandy-Walker Malformations
Variable cystic malformation in posterior fossa, associated with atypical shape and size of posterior fossa + cerebellar hypoplasia.
US Findings:
Enlarged posterior fossa, dilatation of fourth ventricle, dysgenesis of cerebellar vermis and associated hydrocephalus with diverging posterior horns.
Variety of other associated central nervous systems malformations.
Dandy-Walker variant:
Minimal variation of same findings with dysgenesis of vermis and cystic dilatation of fourth ventricle without enlargement of posterior fossa, potentially enlarged posterior fossa CSF space.
3.3.1.5 Corpus Callosum Malformations
Dysgenesis of partial or total agenesis of corpus callosum.
US Findings:
Lack of visualisation of entire or parts of corpus callosum as well as the cingulate gyrus.
Radial diverging sulci reaching to roof of third ventricle (Fig. 3.14).
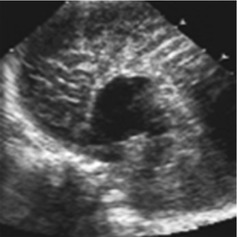
Fig. 3.14
Agenesis of corpus callosum. Sagittal view: no corpus callosum seen. Gyri reach roof of third ventricle, no gyrus cingulum; additional CDS and duplex flow spectrum of pericallosal artery which also runs directly on roof of third ventricle
Atypical impression of frontal horns of lateral ventricle: lateralised, steerhorn shape in coronal view.
Colpocephalic configuration of posterior horns. Elevation of roof of third ventricle.
Minimal forms: only regional thinning or discontinuity of corpus callosum.
Associated malformations, for example: arachnoid cyst, Arnold Chiari and various syndromes (Trisomia 8, 13, etc.).
NOTE: Subtle partial agenesis or dysgenesis of corpus callosum more difficult to visualise on US. Clue to diagnosis – atypical form of cingulate gyrus in respective area, close contact of radially diverging sulci – use coronal view.
3.3.1.6 Lipoma
Typically at corpus callosum or in midline at roof of third ventricle, not associated with other callosal malformations.
US Appearance:
Echogenic space-occupying lesion.
3.3.2 Migration and Gyration Alterations and Disturbances
General Remarks
Any disruption of normal radiating migration of neurons from germinal matrix to peripheral cortex (caused by infection, ischemia metabolic reasons, etc.) can cause severe malformations and clinical sequelae:
Most sensitive imaging technique for assessing migration disturbances, heterotopia and similar phenomena: MRI.
US used as initial tool, may depict gross changes both of gyration as well as echotexture of affected parenchyma – indicating further imaging.
Many more malformations and anomalies exist than mentioned – not listed below as either rare (e.g. brain stem discontinuation, Fig. 3.15a) or sonographically difficult or impossible to diagnose.
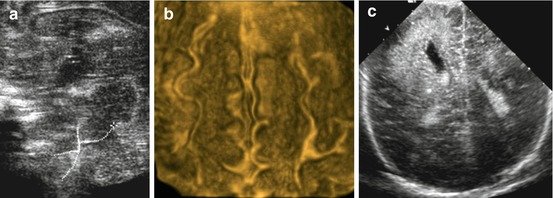
Fig. 3.15
US in brain malformations. (a) Zoomed sagittal midline view: brain stem discontinuity (dotted line). (b) 3DUS surface rendering: gyration becomes conspicuously visible improving US potential to depict and demonstrate gyration disorders. (c) Coronal view: right-sided hemimegalencephaly depicted as focally enlarged and clubbed lateral ventricle with atypical structured and hyperechoic brain parenchyma
3.3.2.1 Lissencephaly, Pachygyria, Macro- or Polygyria and Colpocephaly
Definition and US Findings:
Disruption of normal gyration, focally or more extensive.
Can be completely missing (agyria) or altered, showing many tiny gyri (polymicrogyria), few unusually flat gyri (pachygyria) or enlarged gyri (macrogyria).
Various forms can be combined with varying severity.
Usually cortex altered in area of gyration disorder: may find dilated lateral sulcus, enlarged supratentorial ventricular system and unusual position of prominent posterior horns (colpocephaly).
3.3.2.2 Megalencephaly
Regional disturbance of brain development, can be generalised or focal/unilateral (hemimegalencephaly) and may be associated with metabolic disturbances.
US Findings (Fig. 3.15c):
If unilateral – asymmetric brain configuration with enlargement of affected region/hemisphere.
Pachygyria, thickened cortex and inhomogenous echogenic disruption of normal parenchymal echotexture.
Asymmetry of ventricles with dilatation of the affected side.
Midline shift towards non-affected side may be present.
In generalised form – symmetric appearance of above described findings.
3.3.2.3 Schizencephaly
Gap in brain parenchyma connecting ventricle to extra-axial CSF system.
If filled with CSF – open lip schizencephaly. No fluid separating the lips – closed lip schizencephaly.
US Findings:
Schizencephalic defect seen if CSF filled – anechoic CSF connection between internal and external CSF spaces (Fig. 3.16).
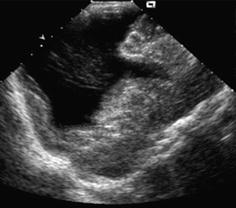
Fig. 3.16
Schizencephaly. Parasagittal view depicts connection between lateral ventricle and external CSF space. Note: Outlining of cleft with cortical gray matter
Close lip schizencephaly – harder to diagnose, meticulous observation of course of cortex and gyri which will not be grossly disrupted by gap, but cortex continues outlining gap borders to wall of ventricle.
Usually associated hypoplasia of affected hemisphere and ventricular asymmetry of affected side.
DDx:
Any kind of porencephalic or cystic defect connecting with ventricle, heterotopia.
NOTE: Cysts usually do not show outlining by cortical grey matter, may connect with (external) CSF space and are often positioned within normal brain, whereas schizencephaly is often associated with other malformations (e.g. hypoplasia of corpus callosum).
3.3.2.4 Holoprosencephaly
Rare severe malformation based on lack of hemispheric differentiation of early fetal brain (Fig. 3.17). Several forms:
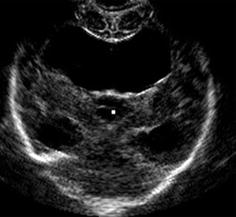

Fig. 3.17
(Semi-)lobar holoprosencephaly. Single ventricle without septum pellucidum in coronal view
Alobar Holoprosencephaly
US Findings:
Single ventricle positioned caudally in midline forming large cystic space. Thalami fused in midline.
No third ventricle depictable: no interhemispheric fissure, no falx, no corpus callosum and no septum pellucidum. There can be cyclopia (the two eyes fused to single eye).
Supratentorial brain consists of single undivided mass.
CDS: only one ACA (azygos ACA).
Semilobar Holoprosencephaly
A slightly milder manifestation.
US Findings:
Smaller (still enlarged) single ventricle in midline with remnant of occipital/temporal horn + respective brain parenchyma.
Rudimentary falx cerebri/interhemispheric fissure seen posteriorly, remnant of third ventricle.
Thalami only partially fused, septum pellucidum absent.
Corpus callosum partially hypoplastic, partially absent.
Lobar Holoprosencephaly
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree