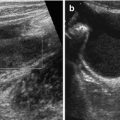
Fig. 6.1
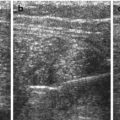
Diaphragm and diaphragmatic motion: (a) Normal diaphragmatic respiratory motion on M-Mode – the echogenic border represents air-filled base of lung, not diaphragm itself, the inhomogeneous spots are minimal peripheral atelectatic areas. (b) No diaphragmatic motion, conspicuously documented by M-Mode, after surgery and postoperative pleural effusion in diaphragmatic palsy. (c) M-Mode under respirator therapy: M-Mode trace reflects effect of mechanical ventilation and not patients’ own respiratory motion
6.2.5 Lung
Normal lung is aerated and only seen indirectly by surface (echogenic structure with reverberation echoes that change with respiration)
Parts beyond aerated lung surface not visualised
NOTE: As soon as US can penetrate lung tissue, some pathology must be expected (e.g. atelectasis, consolidation, effusion, other non-aerated space-occupying process).
Respiratory motion of lung surface used to differentiate normal aerated lung from pneumothorax, where no motion of reflecting surface/air space can be noted:
Also seen in air-filled bronchogenic cysts and severe obstructive hyperinflation
Documentation by video clip or M-Mode
Basal parts of lungs best seen by transabdominal access:
Should be part of any standard abdominal US (as effusion, atelectasis and pneumonia may cause abdominal complains, particularly in young children)
6.2.6 Mediastinum
6.2.6.1 Anterior Mediastinum/Thymus
Mainly Thymus (Fig. 6.2):
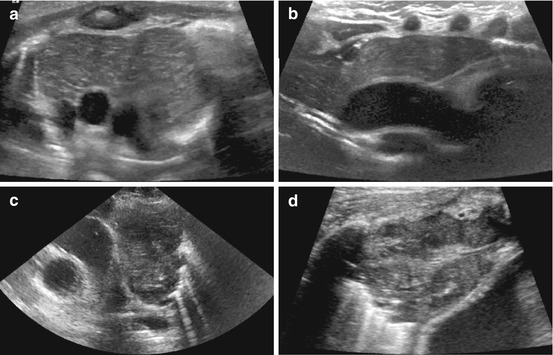

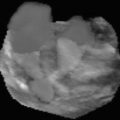
Fig. 6.2
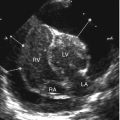
Thymus: (a) Anterior mediastinum, axial section, linear transducer: Large neonatal thymus, serving as window to deeper structures such as the great vessels. Note non-ossified sternum with central ossification centre. (b) Sagittal section, anterior and middle mediastinum, linear transducer in trapezoid format, paramedian view: Large neonatal thymus. Note anechoic non-ossified parts of ribs and large, uncompressed vessels; behind one can see a feeding tube in the oesophagus. (c) Left anterior mediastinum, axial section, sector transducer: Enlarged thymus with inhomogeneous echogenicity in a child with Hodgkin lymphoma. (d) Right anterior mediastinum, axial section, linear transducer in trapezoid format: US in mediastinitis, abscess-like pseudotumorous inflammatory lesions with nodular appearance in the mediastinum
Physiologically large in neonates, then eventually regresses
Shape and size variable
Echogenicity: hypoechoic, mixed, with some septa (“dot-dash pattern”)
Behaviour of soft tissue: not compressing or displacing other structures, particularly vessels
Size of thymus difficult to assess, reliable age-related normal values not available
CDS: some internal vascularity
Value of US:
Differentiate from other mediastinal or chest masses (unclear opacification on chest film)
Demonstrate normal echogenicity and behaviour in relation to surrounding structures of a large thymus
Additionally: ideal acoustic window to deeper structures
NOTE: Large thymus at unusual age may point at diffuse infiltration or thymus hyperplasia; infiltration and tumours will cause increased stiffness and thus subsequent impression or displacement of surrounding structures or crossing vessels.
6.2.6.2 Middle Mediastinum
Contains – among others – large vessels, trachea, potential nodes may be visualised by US (Fig. 6.3):
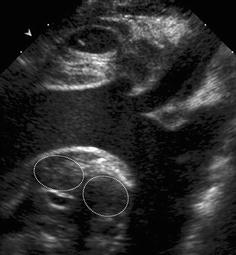

Fig. 6.3
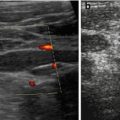
Middle mediastinum: vessels and lymph nodes. Parasternal (jugular) sagittal view, sector transducer: thoracic aortic arch, supra-aortic vessels, two enlarged mediastinal lymph nodes (dotted circular lines)
Particularly feasible in neonates and infants
Large space-occupying lesions, tumours or lymph node enlargement visible
Anatomy of large vessels addressed with echocardiography
6.2.6.3 Posterior Mediastinum
Difficult to visualise by US
Usually anterior access supplemented by posterior paravertebral access
Used for assessing tumours, particularly neuroblastoma
6.2.7 CDS
Except for assessment of vessels, CDS not very useful in normal situation
Indications for chest CDS for DDx in pathology mostly of the lung: e.g. abscess or necrosis, tumour vascularisation, vascular malformations, suspected particularly peripheral pulmonary artery embolism (PAE, resemble triangular subpleural pneumonic areas without depictable vascularistion), etc.
6.3 Pathology of Chest Wall
6.3.1 Aplasia, Variations of Ribs
Quite common, cartilaginous part nicely assessed by US, wide range of rib anomalies:
3DUS reconstructions improve understanding and visualisation (see Fig. 1.34)
Plain film: US complements plain film
6.3.2 Congenital Malformations
6.3.3 Traumatic Changes
Fractures of ribs and sternum: see musculoskeletal US (Chap. 11):
Particularly in cartilaginous parts and sternum
US may be superior to plain film, where these structures are difficult to assess if not significantly displaced
NOTE: Follow entire structure in longitudinal and axial sections to detect any surface interruption/irregularity.
Often some reactive focal subperiosteal haematoma:
Without history, differentiation from osteomyelitis difficult
Particularly if bilateral, multiple, of different age – NAI should be considered.
Additional findings:
Complicated haemorrhagic pleural effusion, atelectasis
Haematoma: seen in all chest wall spaces, usually no indication for imaging – only in unclear cases, complicated course, suspicion of infection (DDx: seroma, etc.)
6.3.4 Chest Wall Tumours
6.3.4.1 Lymphangioma (veno-lymphatic vascular malformation)
US finding: Multicystic space occupying lesions with echogenic septae
Spontaneous haemorrhages with fluid-fluid levels often present (see Fig. 4.12)
CDS: potentially some vessels within septae
6.3.4.2 Lipoma
US finding: Usually slightly inhomogeneous, echogenic mass, sharp margins (Fig. 6.4)
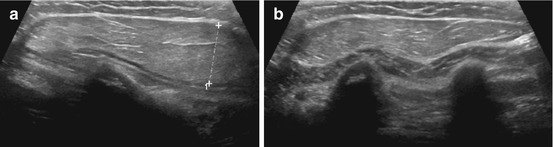

Fig. 6.4
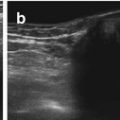
Chest wall lipoma: (a) Chest wall lipoma: well-defined subcutaneous mass (+ +), fat-like intermediate density echoes. (b) Below lipoma mass, structures of chest wall can be appreciated: muscle and ossified ribs (shadowing)
6.3.4.3 Fibroma/Neurofibroma
US finding: Usually sharp margin, hyperechoic or inhomogeneous
6.3.4.4 Other Tumours
Rare; e.g. rhabdomyosarcoma or Ewing sarcoma (Fig. 6.5):
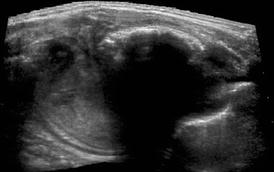

Fig. 6.5
Chest wall tumour – Askin tumour/Ewing sarcoma. Extended field of view US demonstrates large chest wall tumour with calcified part (dorsal shadow) arising from partially destructed rib – i.e. Ewing sarcoma
Sometimes difficult to differentiate from myositis ossificans, particularly Askin tumour.
US finding: No specific sonographic features
6.3.5 Breast
Breast US: In childhood of limited importance
Neonates: Transient physiologic swelling, cystic duct ectasia and cysts seen
Secondary infection with abscess formation and haematoma may occur (Fig. 6.6a)
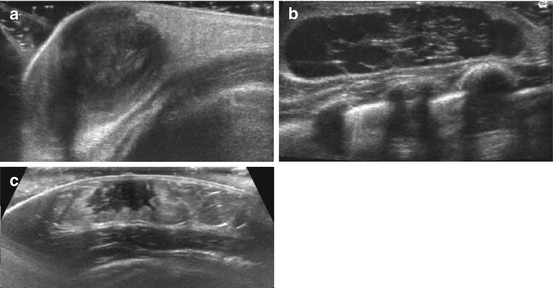
Fig. 6.6
Breast US in childhood: (a) Neonatal breast abscess – huge collection with membrane and adjacent soft tissue reaction (hyperechoic, swelling) after neonatal mastitis; note plenty US gel to facilitate transducer coupling to tissue without interposing air. (b) Impressive cystiform duct ectasia in a breast feed infant. (c) Asymmetric prominent breast tissue at onset of pubarche in 11-year-old girl
(Pre-)puberty cysts, tubular duct ectasia, fibroadenoma, inflammatory formations (Fig. 6.6b):
Overall appearance varies with age and maturation (Fig. 6.6c)
Most pathological entities do not differ from typical US appearance in adults
Additional application of breast US in childhood: Assessment of sexual maturation, documenting presence and size of breast tissue
In girls with suspected hormonal or genetic pathology
In boys with gynaecomastia
In some centres proof of significant breast tissue necessary for treatment decision
NOTE: Breast carcinoma extremely rare in childhood.
CDS: Can be helpful for assessment of superficial tumours or vascular malformations and other pathology described in respective chapters
6.3.6 Role of US and Additional Imaging
US: Supplementary tool in clinically equivocal situation, follow-up
Additional Investigations:
Suspicion of tumour – depending on oncology protocols – plain film, CT/MRI
Assessment of osseous structures: plain film, rarely CT
Mammography: rarely indicated, and only in/after puberty
6.4 Pathology of Pleural Space
6.4.1 Pleural Effusion
Definition: Some fluid in between two pleural sheets of varying aetiology:
Cardiac, inflammation, trauma, tumour, etc.
Most common pleural change, most common indication for chest US
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree