Always assess adjacent spaces: subphrenic and subsplenic/perisplenic
CDS helpful when looking for vessel pathology (e.g. posttraumatic aneurysm, collateral and enlarged veins in portal hypertension, thrombosis/infarction, etc.)
8.1.5 Normal Anatomy
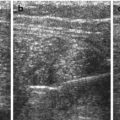
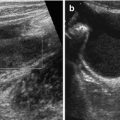
Normal spleen – inverted comma shape, larger surface positioned adjacent to left diaphragm.
Large parallel vessels seen in hilus – follow into organ and to midline along pancreas.
Homogeneous organ of rather low echogenicity with smooth margins and rounded poles (Fig. 8.1).
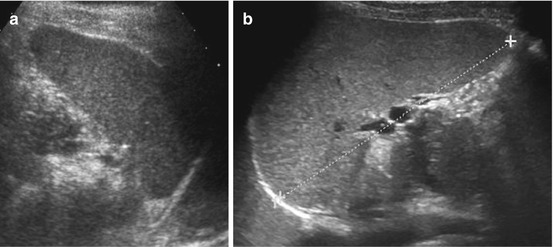

Fig. 8.1
Normal spleen. (a) Axial view. (b) Longitudinal view, with standardised section for measurement (+ +): adequate section defined by hilar vessels, lower pole and upper pole (medio-posterior)
Size – see age-adapted normal values (Table 8.1), as rule of thumb: maximum length (cm) = 6 + 1/3 of age (years). Measurements taken in reproducible section that includes hilar vessels:
If spleen reaches lower third of normal sized and positioned left kidney = enlarged
In neonates shape and position of spleen different (more oblique)
8.1.6 Normal Variants
8.1.6.1 Splenunculus (Accessory Spleen)
Most commonly close to hilus (may be positioned anywhere), usually spheric or ovoid, same echogenicity as splenic parenchyma, often some connection to spleen with vascular hilus (arises from splenic vessels) (Fig. 8.2).
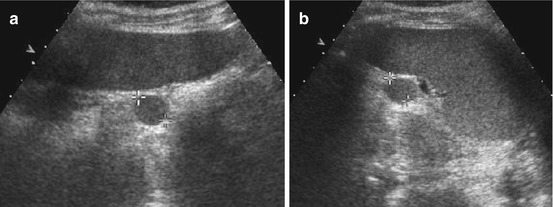

Fig. 8.2
Splenunculus/splenule (accessory spleens). Small splenules in two different cases (+ +) documented in axial (a) and longitudinal view (b)
NOTE: Splenunculi may cause acute abdomen by torsion and infarction, often notable by lack of CDS signals. Sometimes difficult to differentiate from lymph nodes (or real accessory spleen/polysplenia).
8.1.6.2 Splenic Lobulations and Clefts
Important to avoid confusion with adrenal or renal mass, scars or traumatic lesions.
8.1.7 Malformations
8.1.7.1 Asplenia
Definition
No spleen, rare, associated with other systemic malformations and syndromes.
US Finding
No spleen/splenunculus seen.
NOTE: Thorough assess entire abdomen for small/ectopic spleens.
8.1.7.2 Polysplenia Syndrome
Definition
No normal single spleen, but multiple smaller organs, may be positioned in normal left upper quadrant or ectopic, often mobile and prone to torsion and infarction.
Rare variant: right-sided position – may be confusing. Shape can vary.
US findings same as in normal spleen.
NOTE: In inverted abdominal or systemic situs, liver is positioned on left side and spleen in right upper quadrant, often associated with spleen anomalies and variations.
Associated abdominal anomalies: disruption of inferior vena cava, azygos continuation, pancreas variations, bowel malrotation and renal agenesis/hypoplasia. Additionally there may be thoracic and cardiac anomalies.
8.1.7.3 Wandering Spleen
Definition
Slightly atypical shape, not normally fixed by gastrosplenic/spleno-renal ligament, commonly positioned ectopically. Increased risk of torsion and infarction.
US findings same as in normal spleen.
8.1.8 Splenomegaly
Definition
Increased size – see normal values.
Caused by infection (acute or chronic, bacterial or viral, other rarer organisms), congestive in portal hypertension and cardiac insufficiency, haemolytic anaemia and infiltration/depositions in systemic and metabolic disease (e.g. mucopolysaccharidosis, glycogenosis, leukaemia, lymphoma).
Secondary to (large) cysts and abscesses.
US Findings
Depending on underlying disease, parenchymal structure normal or disrupted. May be patchy and inhomogeneous.
Echogenicity mostly normal, may be decreased or increased. Sometimes inhomogeneous.
In significantly enlarged spleens, extended view techniques are helpful to measure entire length; 3DUS reported to be most accurate for volume assessment (Fig. 8.3).
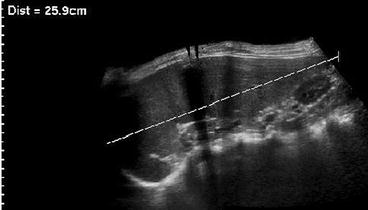

Fig. 8.3
Extended field of view in splenomegaly. Significantly enlarged spleen only measurable using extended field of view US
NOTE: In splenomegaly carefully assess for potentially underlying causes (e.g. liver/portal vein pathology and lymphoma/leukaemia) or additional findings (e.g., ascites and gall stone).
8.1.9 Trauma
Definition
Spleen injury common in blunt abdominal trauma in children and may lead to acute haemorrhage.
Children often compensate even severe haemorrhage for significant time to eventually deteriorate suddenly, making a thorough US investigation particularly valuable. Grading is available and should also be attempted when diagnosed by US, as grade correlates with risk of complications (Table 8.2).
Table 8.2
Splenic injury grading system
Grade I |
Subcapsular haematoma <10 % of surface area |
Capsular laceration <1 cm depth |
Grade II |
Subcapsular haematoma 10–50 % of surface area |
Intraparenchymal haematoma <5 cm in diameter |
Laceration 1–3 cm depth not involving trabecular vessels |
Grade III |
Subcapsular haematoma >50 % of surface area or expanding |
Intraparenchymal haematoma >5 cm or expanding |
Laceration >3 cm depth or involving trabecular vessels |
Ruptured subcapsular or parenchymal haematoma |
Grade IV |
Laceration involving segmental or hilar vessels with major devascularisation (>25 % of spleen) |
Grade V |
Shattered spleen |
Hilar vascular injury with devascularised spleen |
US Findings
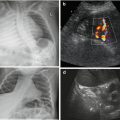
Potentially increased size due to subcapsular haematoma, which in early posttraumatic phase sometimes cannot be discriminated from normal spleen tissue; only perisplenic fluid or later phases with sedimentation of erythrocytes and complex fluid-like appearance of haematoma enables visualisation (Fig. 8.4a, b).
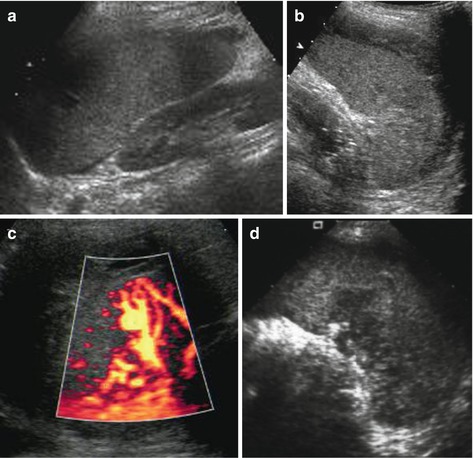

Fig. 8.4
Spleen hematoma. A series of images demonstrating various appearances of acute splenic trauma: only slight subcapsular/perisplenic fluid (a, b) with poorly depictable and hazy parenchymal lesion, regional perfusion alteration without depictable parenchymal changes but subcapsular haemorrhage (c) and obvious parenchymal damage (d)
Contusions without haemorrhage often only exhibit regionally more inhomogeneous structure without demarcation of haematoma
In laceration contour disrupted – complex shape, disruption of parenchyma, may reach central vessels
Reduced diaphragmatic motion (reflexive-reactive) – helpful indirect sign – potentially follow-up exam after 6–24 h
Normalisation of tissue will take weeks to months; gradually lesion becomes increasingly hypoechoic and demarcated/cyst like (Fig. 8.5); secondary posttraumatic cysts or fibrous scar tissue may remain.
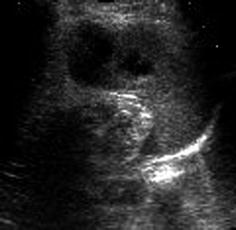

Fig. 8.5
Old splenic hematoma. Old splenic trauma: slight subcapsular fluid with cyst-like necrotic parenchymal areas after laceration
NOTE: Peritoneal fluid only initially located perisplenic will distribute in entire abdominal cavity depending on position although originating from spleen – thus location of intra-abdominal fluid does not necessarily correlate with origin/site of injury.
CDS
Useful to assess vitality or areas with lack of perfusion, e.g. by disruption or infarction
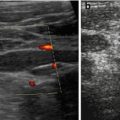
In follow-up useful to detect posttraumatic aneurysms or AVF, which may rupture and cause secondary haemorrhage (Fig. 8.6)
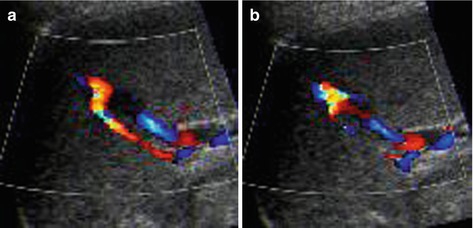

Fig. 8.6
Splenic posttraumatic AVF. (a) Splenic posttraumatic AVF on CDS, with obvious depiction of the red-coded feeding artery. (b) CDS depicts aliasing at the AVF and demonstrates the draining vein encoded in blue
Role of US
Therapeutically splenic injury is increasingly managed conservatively or with techniques that enable salvage of some splenic tissue. Thus imaging diagnosis, and follow-up of splenic injury is particularly important.
Initially US most essential for depiction of free abdominal fluid (FAST) – indication for surgery usually based on clinical grounds and indication for additional imaging usually based on trauma history and severity, complemented by US findings.
NOTE: In early phase US may miss even significant splenic injury, unless aCDS or ce-US is performed.
8.1.10 Splenic Infarction
Definition
Rare condition in children, most commonly associated with sickle cell anaemia.
Other causes: acute portal vein thrombosis (venous infarction), infiltration, inflammation and trauma.
US Findings
Acute enlargement of spleen, initially even with normal echotexture. Secondary heterogeneous inhomogenously patchy parenchymal echoes due to haemorrhage and haemorrhagic infarction (Fig. 8.7a). Increasingly hypoechoic-cystic aspect on delayed imaging (Fig. 8.7b).
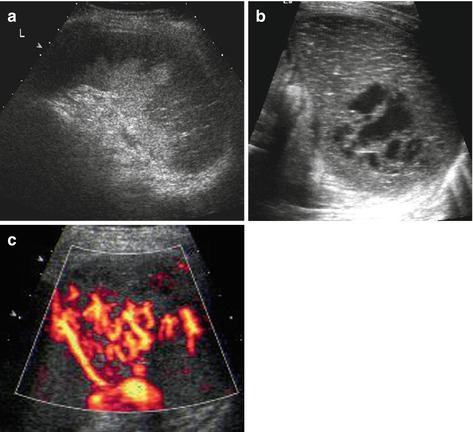

Fig. 8.7
Splenic infarction. (a) Subacute splenic infarction in sickle cell anaemia: regional alteration of tissue echogenicity reaching into periphery/subcapsular; some normal spleen tissue left in the centre near the hilus. (b) Old splenic infarction – creates images similar to posttraumatic changes, with cyst-like necrotic area. (c) aCDS demonstrates non-perfused, infarcted areas even in a very early stage, where gray scale findings may be very subtle or even not yet depictable
(a)CDS
Large/main infarcted vessels depictable by CDS
Infarcted splenic tissue, particularly in early phase, often only detectable by aCDS or ce-US (Fig. 8.7c)
8.1.11 Space-Occupying Lesions of the Spleen
8.1.11.1 Cysts
Definition
Congenital – dysontogenetic, part of systemic cystic disease, posttraumatic and postinflammatory (pseudocysts)
Posttraumatic/infectious cysts may remain stable or diminish and resorb. Congenital/systemic splenic cysts may grow concordant to spleen growth or even more. In syndromatous or systemic cystic disease additional cysts may appear
US Findings
Simple cyst: smooth margins, sharp border, thin membrane, no central structures, anechoic fluid, often solitary (Fig. 8.8)
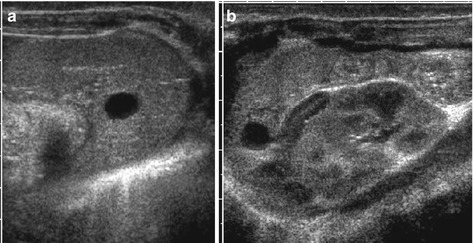

Fig. 8.8
Splenic cyst. (a) Axial and (b) longitudinal view of spleen with dysontogenetic cyst in an infant. Note physiologically prominent adrenal gland and typical appearance of neonatal kidney in (b)
Epidermoid cyst: may contain some echoes, sedimentation phenomena occur
Complex cysts: polylobulated with septae, some irregular margin or thick membrane, potentially nodular components – large variety of underlying conditions to be considered (history? Echinococcosis or other unusual infection?)
NOTE: Use CDS to differentiate vascular pathology from cysts.
Potentially US-guided puncture of cyst for aspiration/diagnosis and therapeutic instillation of various agents.
8.1.11.2 Abscess
Definition
Rare condition in childhood, most commonly observed in immunocompromised patients or posttraumatically.
Most common cause: staphylococcus, salmonella, fungus, and hydatid disease.
US and CDS Finding:
Similar to complicated cyst – spheric space-occupying lesion, some irregular and hazy margin, variable (often hypoechoic) echotexture depending on duration and underlying condition (Fig. 8.9). Usually no proper capsule visible; sometimes perifocal hyperemia seen on aCDS. Potentially halo-like surrounding hypoechogenicity (target sign).
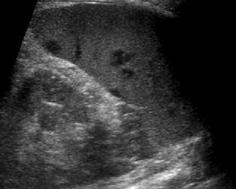

Fig. 8.9
Splenic abscess/infiltration. Multiple hypoechoic spheric/round parenchymal lesions in slightly enlarged spleen – in this case these were fungal abscesses during chemotherapy; however, any other small abscess or infiltration (e.g. in lymphoma or granulomatous disease) may exhibit similar images and is sonographically indistinguishable
May cause secondary infarction of dependent area.
NOTE: Multiple abscesses may be confusing, sometimes cannot be differentiated from necrotic changes after infarction – if no systemic proof for infectious cause found, US-guided puncture and drainage may be an option. But splenic punctures have considerable risk of haemorrhage – take proper precautions.
8.1.11.3 Tumours and Space-Occupying Lesions
Definition
Rare. Haemangioma, hamartoma, and lymphangioma/lymophangiomatosis (e.g. Gorham-Stout disease) occur
Malignant solid tumours other than infiltration in lymphoma/leukaemia extremely rare (e.g. angiosarcoma)
Inflammatory pseudotumour may exhibit calcifications
Involvement in storage disease or systemic granulomatous disease as well as rare (tropical) infection may be difficult to distinguish from tumorous condition
US Finding
Very commonly hypo- or hyperechoic, nodular, space-occupying lesions, sometimes with hyperperfusion (Fig. 8.10)
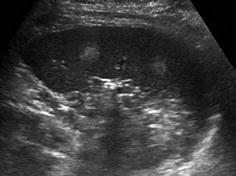

Fig. 8.10
Spleen haemangioma. Two focal round hyperechoic areas within spleen parenchyma consistent with splenic haemangiomas
NOTE: Multinodular irregularities also seen in splenic infiltration by metastatic or systemic disease (e.g. metabolic disease, leukaemia, lymphoma, Langerhans cell histiocytosis and Gorham-Stout disease). Manifestation of other systemic granulomatous disease should be considered for DDx (e.g. tuberculosis, cat scratch disease and sarcoidosis).
8.1.11.4 Role of US
Ideal method for diagnosis and follow-up:
Particularly if ce-US available
Restrictions: in early posttraumatic setting, definition of underlying entity.
Additional/alternate imaging:
Ce-MRI/PET, rarer ce-CT – complementary imaging – optimal for lesion detection (contrast enhanced techniques), also restricted for lesion characterisation/entity definition
Scintigraphy
8.2 Pancreas
8.2.1 Requisites
Preparation:
Fasted patient helpful for easier acoustic access
Measures to avoid gaseous bowel and shadowing by stomach content
Positioning:
Commonly accessed in patient lying supine
Positioning manoeuvres (if tolerated) may be helpful – decubitus and semi-upright
Transducers
Usually curved array for general assessment
Sometimes sector transducer if very small sonographic window
Linear array for detailed assessment, standard in neonates and infants
Frequencies vary with age/necessary penetration depth
8.2.2 Indication
Various conditions that either involve or affect pancreas:
Malformation
Inflammation
Trauma
Obstruction
Cysts, rarely tumours
8.2.3 Course of Investigation
Access via median upper abdomen:
Use left liver lobe as window for head of pancreas and body
Access via left upper quadrant
Get Clinical Tree app for offline access

Use spleen as window to tail (see Fig. 8.11c)
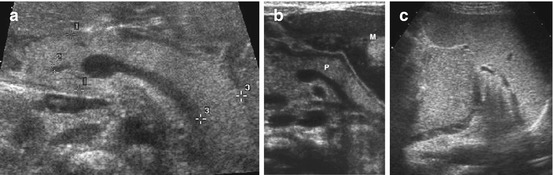
Fig. 8.11
Normal pancreas. (a) Normal neonatal pancreas with homogeneous echogenicity, measurement of head (+ +1), tail (+ +3) and common bile duct (+ +2). Axial view using graded compression and large left liver lobe as US window. (b) Normal pancreas (P), axial view accessed via full stomach (M) after feeding to improve visualisation. (c) Axial transsplenic view to prominent pancreatic tail
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree