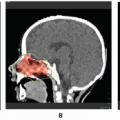
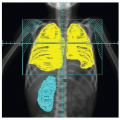
Several subtypes of NHL in children are associated with distinct clinical manifestations and sites of disease (
Table 8.1). Symptoms leading to diagnosis usually are of short duration. Approximately 25% of children with NHL have an anterior mediastinal mass (usually T lymphoblastic or diffuse large B cell) and present with wheezing, stridor, and cough
progressing to dyspnea. The majority of these patients are adolescents, and their presentation may pose a medical emergency (
14). Patients with large anterior mediastinal masses are at risk of cardiac or respiratory arrest during general anesthesia or deep sedation. A careful workup including a chest computed tomography (CT) scan with airway measurements is essential (
15) before attempting any procedures. The least invasive procedure (e.g., biopsy of a peripheral lymph node) should be carried out. If these procedures are not successful in providing a diagnosis, then a CT-guided needle biopsy of the mediastinal mass should be considered (
16). In some clinical situations (e.g., orthopnea or significant airway narrowing), preoperative or preprocedure steroids should be considered for up to 48 hours. The use of steroids has largely replaced localized irradiation in this setting. Primary gastrointestinal involvement occurs in about 30% (usually Burkitt histology), commonly presenting as an abdominal mass with ascites, an “acute abdomen” from an intussusception, or rarely a malnutrition syndrome with colitis symptoms (
6,
17). The majority of children with Burkitt lymphoma presenting with an ileal-cecal intussusception have limited gastrointestinal involvement that is amenable to complete surgical resection (Murphy stage 2 or group A). In 20-30% of children, the head and neck, including the Waldeyer ring or cervical lymph nodes, is the site of origin. The remainder of patients have miscellaneous primary sites, including bone, breast, skin, epidural space, or noncervical lymph nodes (
6). Involvement of the bone marrow at diagnosis occurs in 10-30% of patients with Burkitt and lymphoblastic lymphomas. Overt CNS involvement at diagnosis is not common but is mostly seen in children with advanced-stage Burkitt and lymphoblastic lymphomas. Children who develop Burkitt lymphoma in endemic areas of the world often have a mass in the head or neck region (especially jaw) in contrast to the abdominal presentation typical of nonendemic Burkitt lymphoma.