Luigi Natale, Agostino Meduri
Non-Ischaemic Acquired Heart Disease
Non-ischaemic heart disease (NIHD) accounts for nearly half of the cardiac deaths. This group of diseases is extremely heterogeneous, including cardiomyopathies, valvular diseases, cardiac masses and pericardial diseases. Modern non-invasive imaging techniques have increased diagnostic accuracy for all these diseases, with a consequent decrease in the number of invasive procedures.
Role of Imaging
In the past, NIHD diagnosis was based on chest radiography and invasive angiography; the introduction of echocardiography has greatly modified the diagnostic approach, as both myocardium and heart chambers are visualised non-invasively, in real time and with the same examination. Furthermore, magnetic resonance has increased the role of non-invasive imaging, with a wider field of view, higher contrast resolution and tissue characterisation capabilities, coupled with extremely accurate, operator-independent functional assessment. Finally, multidetector ECG-gated CT has had a deep impact on non-invasive coronary artery imaging; recent technological improvements have also made CT effective in the assessment and therapeutic planning of NIHD, particularly in valve diseases and cardiac masses.
Chest Radiography
Chest radiography (CXR) still remains the first-line examination in both ischaemic and non-ischaemic heart disease. Its advantages are low cost, non-invasiveness, wide availability and unique information on pulmonary haemodynamics; of course, it is extremely non-specific and has a low sensitivity, particularly before disease is at an advanced stage.
CXR interpretation is based on a sequential procedure: the chest wall anatomy may explain modification in heart contours; pleural or parenchymal disease may cause non-specific symptoms such as chest pain. Analysis of vessel size and distribution which reflect the haemodynamic status, is fundamental in assessing heart disease: indeed, size and distribution are strictly related to capillary wedge pressure and pulmonary venous pressure, which equalises left atrial pressure and, consequently, end-diastolic left ventricular pressure (EDLVP). So, depending on the acute or chronic development of the disease, it is possible to obtain a non-invasive assessment of EDLVP.
Furthermore, a general assessment of the presence or absence of cardiomegaly will help to create a differential diagnostic list of possible diseases, with or without cardiomegaly. The next step is the analysis of modifications of cardiac contours in both frontal and lateral views which may be helpful in identifying specific chamber enlargement.
Echocardiography
Echocardiography is the most commonly performed imaging examination in the assessment of NIHD. It is a non-invasive, portable ultrasound technique that allows high-resolution, two- and three-dimensional (2D and 3D) views of the cardiac chambers, valves and pericardium. This technique, with either a transthoracic or transoesophageal approach, can assess cardiac anatomy and ventricular function. When combined with Doppler and colour-Doppler techniques, valvular regurgitation and stenosis and transvalvular pressure gradients can also be assessed.
Magnetic Resonance Imaging
Cardiac magnetic resonance imaging (CMR) is rapidly becoming very useful in the assessment of NIHD. Its role is most valuable in (1) serial measurement of ventricular function in patients with cardiomyopathy (considered superior to echocardiography in reproducibility); (2) evaluation and quantification of valve function, including stenosis and regurgitation; (3) morphology and extent of involvement in cardiac tumours; and (4) value of post-contrast delayed enhancement in assessing diagnosis and determining prognosis in many diseases, such as hypertrophic cardiomyopathy, dilated cardiomyopathy (DCM) and many types of infiltrative myocardial diseases including sarcoidosis and myocarditis.
Computed Tomography
Until recently, conventional computed tomography (CT) had a limited role in the evaluation of NIHD. The increasing use of ECG-gated subsecond multidetector CT techniques has the potential to make cardiac CT a viable alternative in assessing cardiac function. More recently cardiac CT has also been shown to be useful in the assessment of valvular function. However, as yet, CT has no demonstrable advantage over echocardiography or CMR in the evaluation of NIHD. Furthermore, despite recent reconstruction advances, radiation dose remains an issue. While imaging of the coronary arteries has become feasible with exposures below 1 mSv, even higher doses are necessary for functional assessment (full acquisition dose is needed over the complete heart cycle as opposed to prospective gating techniques for imaging of the coronaries).
Cardiomyopathies
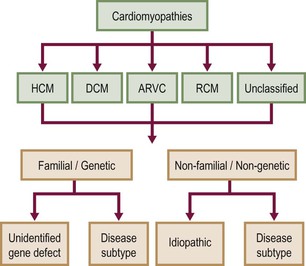
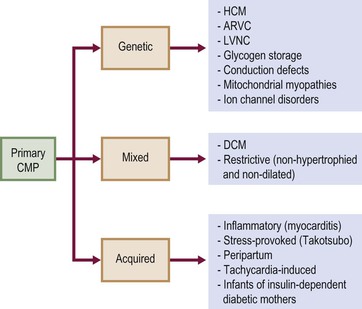
According to the 2006 American Heart Association (AHA) definition,1 cardiomyopathies (CMPs) are ‘a heterogeneous group of diseases associated with mechanical and/or electrical dysfunction, usually exhibiting inappropriate hypertrophy or dilatation, due to a variety of causes, often genetic, confined to the heart or part of systemic disorder’. This classification divided CMPs into primary and secondary; primary CMPs are subdivided into genetic, mixed and predominantly non-genetic and acquired, while secondary CMPs are a variety of diseases that can affect the myocardium (Fig. 21-1).
In 2008, the European Society of Cardiology (ESC) proposed another classification,2 based on different phenotypes (hypertrophic, dilated, etc.) that are subclassified into familial genetic and non-familial non-genetic (Tables 21-1 and 21-2). While the AHA classification is based more on pathology, the ESC classification is more clinical, as, for example, a hypertrophic phenotype can be due to many diseases that cause myocardial thickening—hypertrophic CMP, hypertensive CMP, Fabry disease, amyloidosis, etc. In this chapter the phenotype approach will be used, according to the ESC classification.
TABLE 21-1
Summary of Familial Diseases Causing Cardiomyopathies, by American Heart Association
| Familial | ||||
| HCM | DCM | ARVC | RCM | Unclassified |
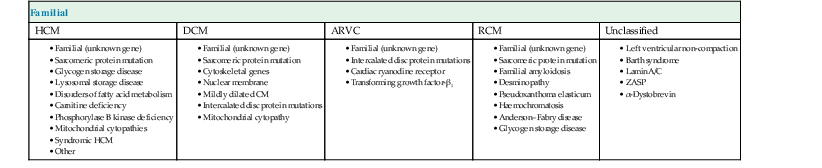
Modified from Maron et al.1
TABLE 21-2
Summary of Non-familial Diseases Causing Cardiomyopathies, by American Heart Association
| HCM | DCM | ARVC | RCM | Unclassified |
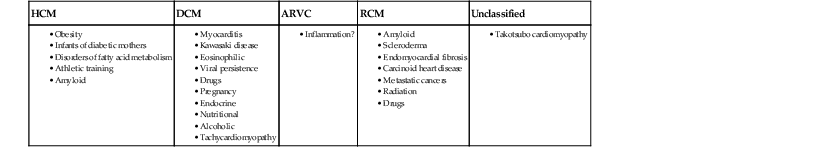
Modified from Maron et al.1
Hypertrophic Pattern
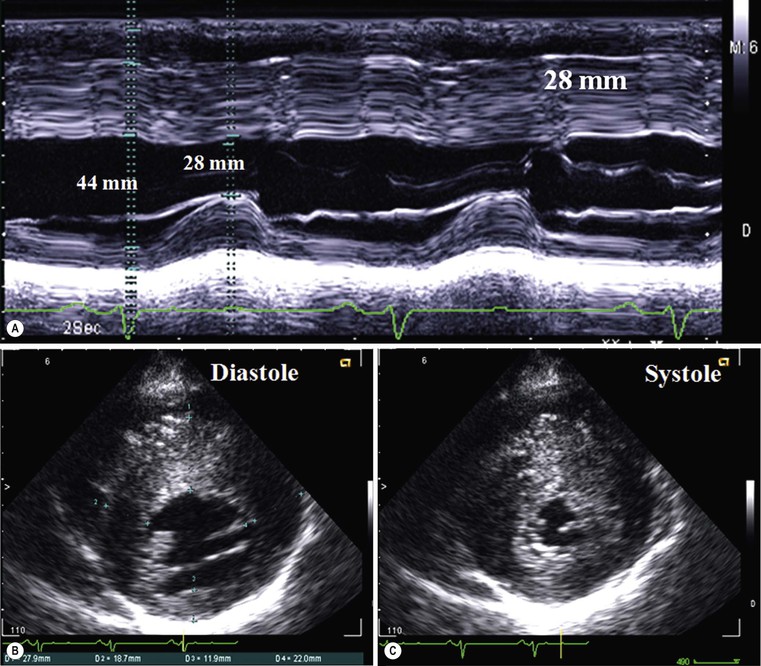
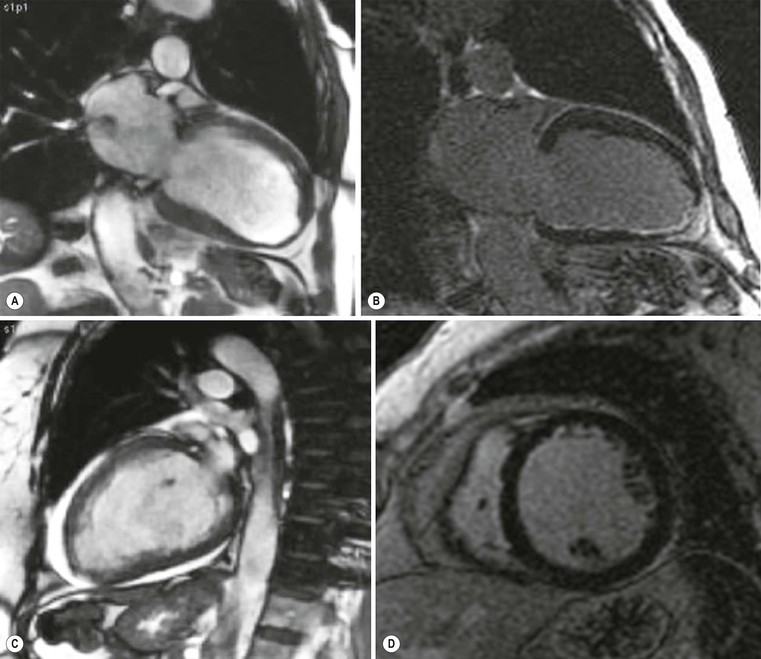
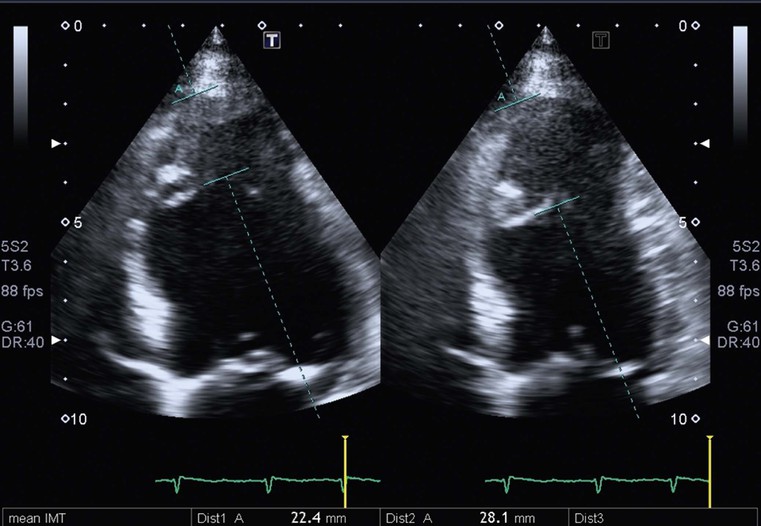
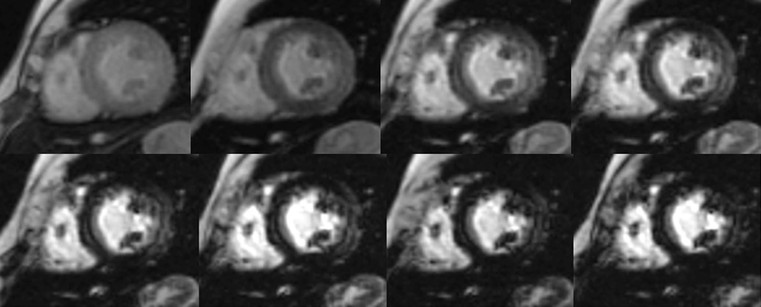
Increased myocardial thickness can be due to a variety of diseases, both familial and non-familial; among the familial, the classical hypertrophic cardiomyopathy (HCM) is the most common. It is autosomal dominant, and is defined as a sarcomere disease (with a number of different mutations), characterised by an excessive hypertrophy of the myocardium (not explained by other causes) in a non-dilated left ventricle.1 Eleven mutations have been recognised with the most common affecting the β-myosin heavy chain. Pathologically there is disarray of myocytes with a variable amount of interstitial fibrosis, caused by microvascular bed damage. Typically, increased septal thickness, exceeding 15 mm, is recognised at echocardiography; in the presence of ECG abnormalities and symptoms (such as chest pain, shortness of breath and dizziness, but also pre-syncope, syncope and arrhythmias), interventricular septum increased thickness is sufficient for diagnosis. Another echocardiographic criterion is a ratio between septal thickness and inferior wall of left ventricle at midventricular level higher than 1.3.3
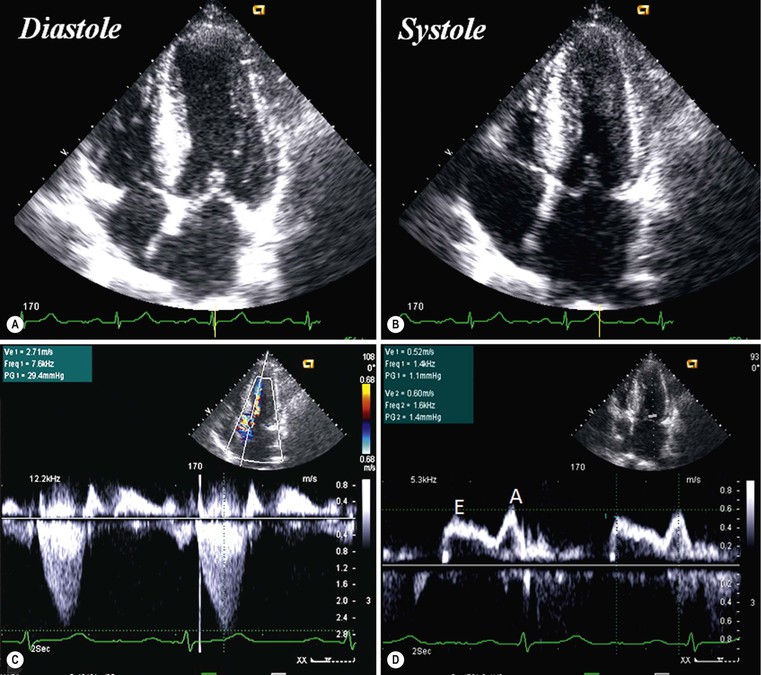
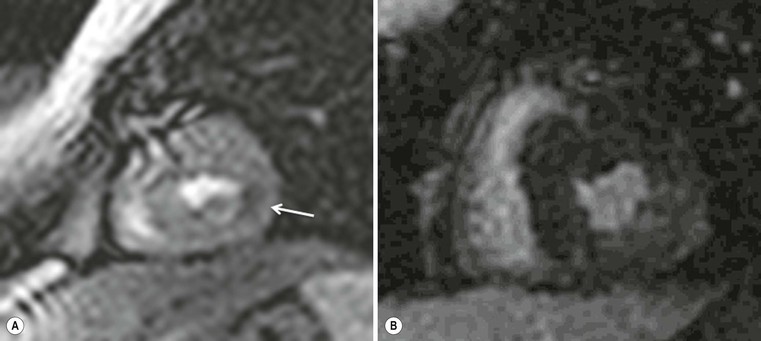
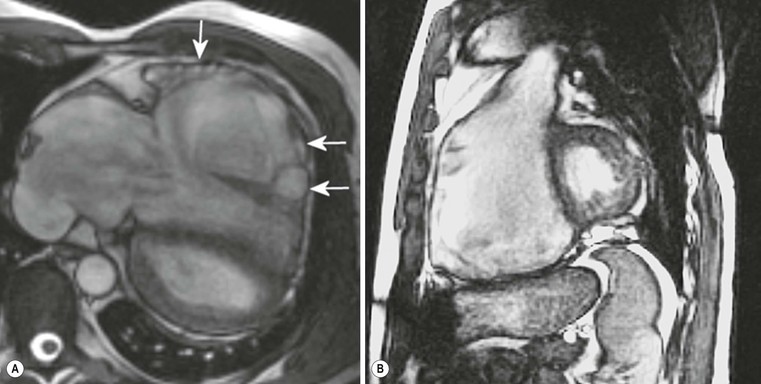
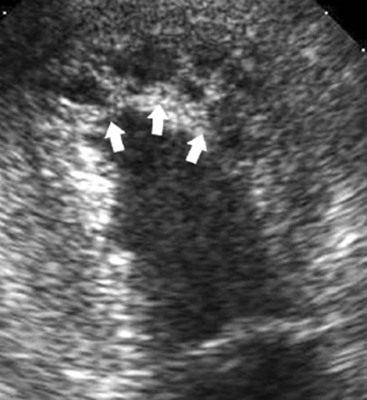
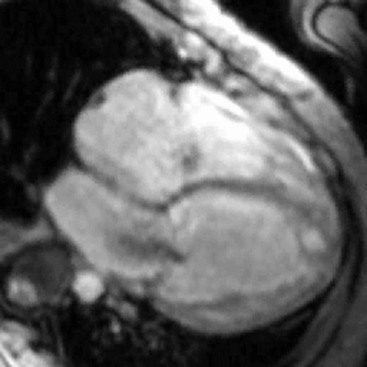
CXR is often unhelpful: in concentric hypertrophy there may be a rounded third left cardiac contour, different from that due to aortic stenosis and systemic hypertension, which can both cause concentric hypertrophy of the left ventricle. Echocardiography can easily assess myocardial thickness (Fig. 21-2); however, there are many patterns of hypertrophy distribution, not all of them easily recognisable by ultrasound. Hypertrophy can be asymmetrical or septal, with or without left ventricle obstruction, symmetric, apical, midventricular, mass-like and non-contiguous.4 In asymmetrical or septal forms, 25% of cases show a systolic obstruction of the left ventricle outflow tract (Fig. 21-3), due to the movement of the anterior leaflet of the mitral valve towards the hypertrophic interventricular septum, caused by the Venturi effect. This obstruction can be present at rest or only during/following physical exercise; it can be easily recognised at echocardiography and echo-Doppler.
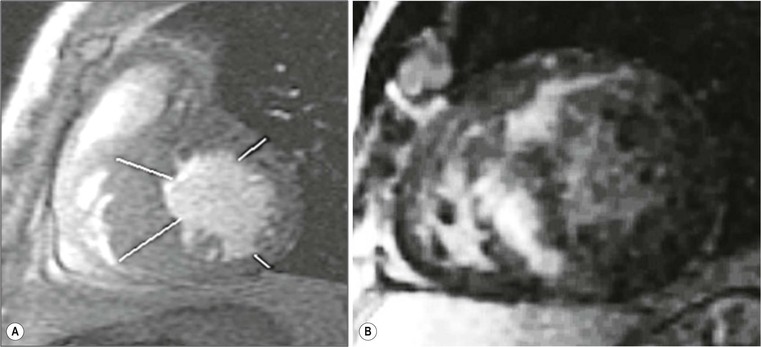
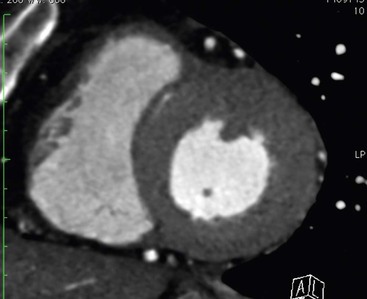
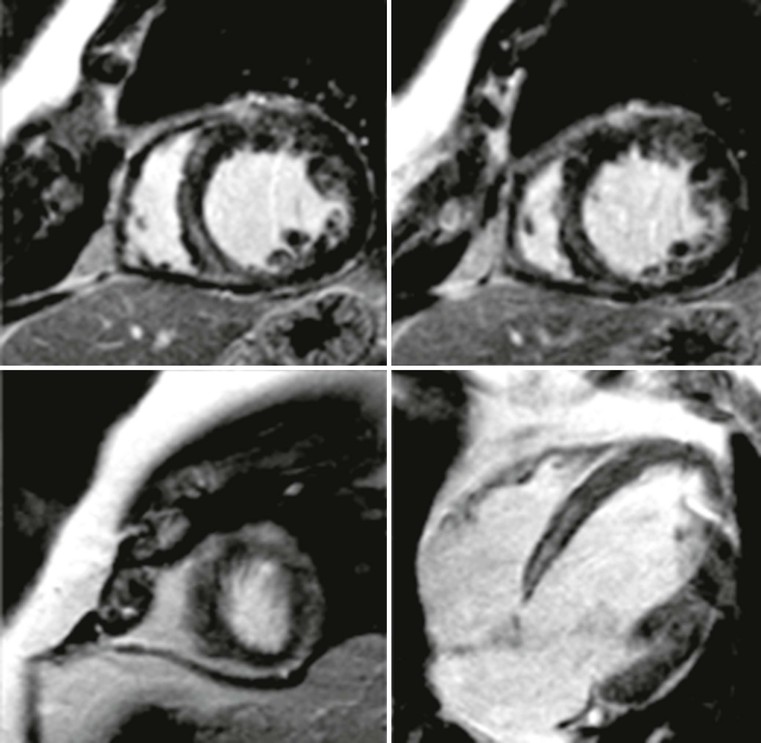
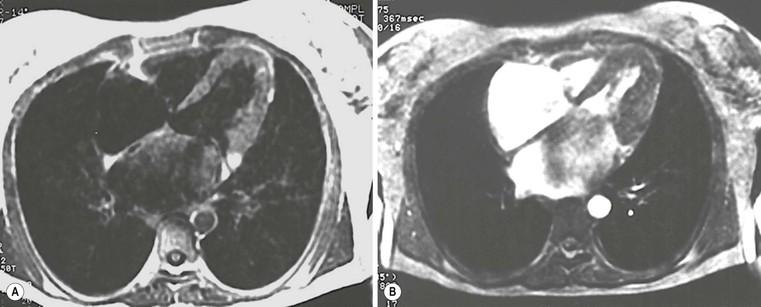
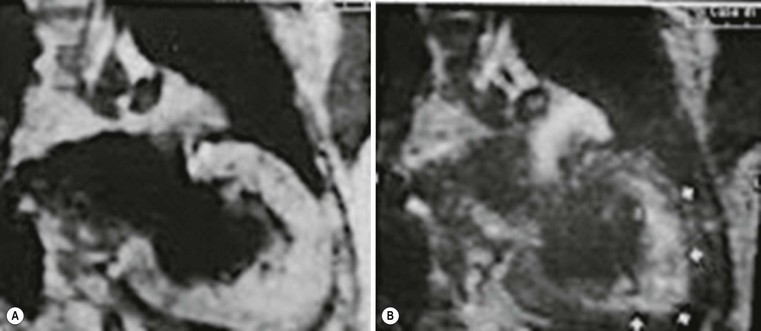
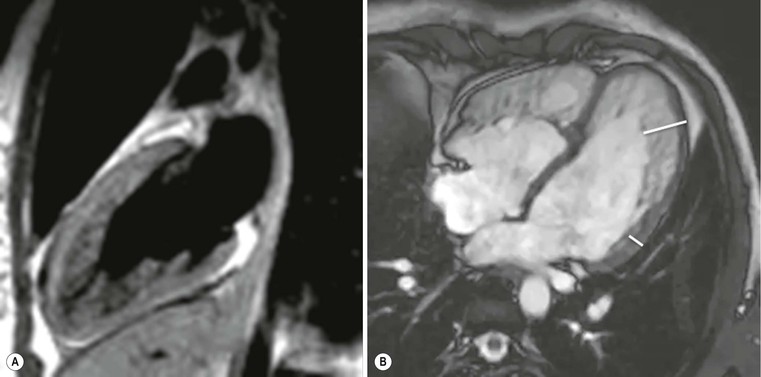
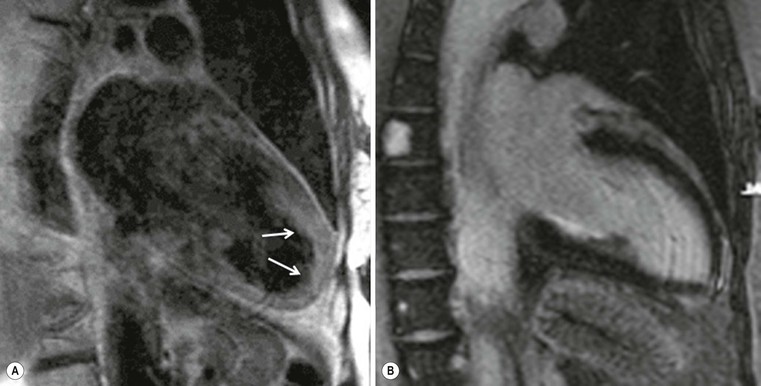
Echocardiography has some limitations,5 particularly in apical forms close to the low-frequency probe, and in mass-like forms where differential diagnosis with tumours can be difficult. In all these cases, and especially when the acoustic window is poor, MRI is extremely useful and accurate;4 it provides precise measurement of wall thickness (Fig. 21-4), is more accurate in left ventricle mass quantification and can easily detect and quantify right ventricle involvement. Such morphological information can also be easily obtained by cardiac CT6 (Fig. 21-5); modern CT equipment provides this information at a very low dose (1–3 mSv). However, the most relevant information is provided by MRI and refers to the presence of interstitial fibrosis; by means of the analysis of delayed enhancement after administration of contrast agent, fibrotic tissue can be easily detected as an area of ‘bright’ myocardium, due to the increased extravascular bed of collagen and its impaired washout, compared with normal ‘dark’ myocardium.7 The distribution of delayed enhancement can be either diffuse, with elective localisation in the septum and relative sparing of subendocardial layer (different myocardial infarct scar) or at anterior and inferior septal insertion, or patchy, with large foci of intramural enhancement8 (Fig. 21-5B).
Detection of fibrosis is extremely important, as it is strictly related to prognosis: a variety of published papers have reported the incidence of severe arrhythmias, due to re-entry mechanisms, and sudden cardiac death in young patients (<40 years) or progression to heart failure in older patients (>40 years) with HCM and severe fibrosis demonstrated at MRI.9–11
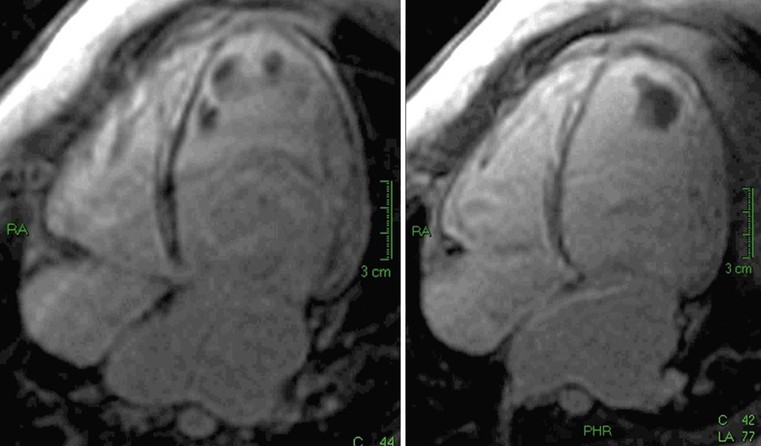
MRI can demonstrate that fibrotic tissue is probably due to impairment of intramural myocardial blood supply;12 in fact, in the case of acute chest pain it is possible to see focal intramural areas of oedema (acute damage with non-ischaemic pattern) and vasodilator stress perfusion MRI can demonstrate a reduced myocardial flow reserve, corresponding to areas of fibrosis during late enhancement (Fig. 21-6). Finally, MRI permits recognition of associated findings, such as mitral regurgitation, and more sophisticated functional evaluation may reveal impairment of diastolic filling and radial or circumferential strain.
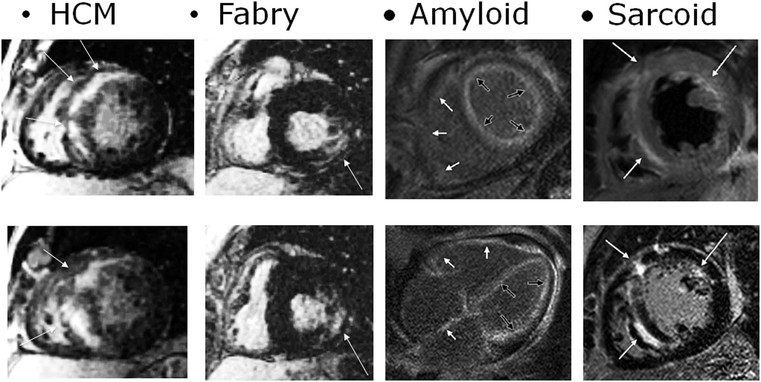
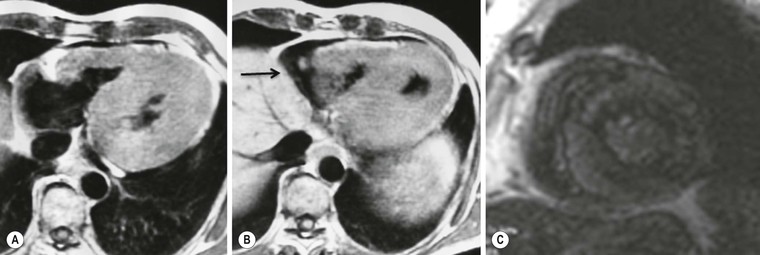
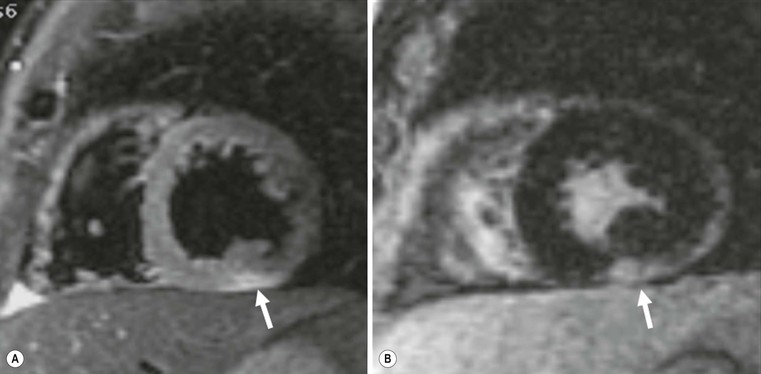
The hypertrophic phenotype that is increased myocardial thickness can be due to other cardiomyopathies; delayed enhanced MRI is particularly useful in the differential diagnosis,8 particularly for storage disease such as Anderson–Fabry disease,13 amyloidosis14 and granulomatous non-infectious diseases, e.g. sarcoidosis.15,16 In these cases, delayed enhancement patterns are different from those of HCM (Fig. 21-7); in Anderson–Fabry disease it is typically subepicardial in the lateral wall, with different presentations in amyloidosis. Due to the diffuse infiltration by amyloid and its link to gadolinium compounds, it is difficult to null myocardial signal, resulting in diffuse intermediate to bright signal intensity; alternatively, a patchy intramural pattern can be present, with small bright spots. Also sarcoidosis can present with the hypertrophic phenotype; again, a patchy pattern of distribution is more frequently present, with small foci, reflecting interstitial distribution of granulomas.
Dilated Phenotype
Dilated cardiomyopathy (DCM) is defined as a left ventricular dilatation with systolic dysfunction not caused by abnormal loading (as in hypertension or valve disease) or coronary artery disease.2 A dilated phenotype can have many different causes; among familial forms, autosomal dominant ones are more frequent, due to mutations of cytoskeletal, sarcomeric and other protein genes. Other forms are X-linked, such as muscular dystrophy. Non-familial DCMs include end-stage inflammatory diseases (infective and non-infective myocarditis), nutritional deficiencies, endocrine dysfunctions and drug toxicity.
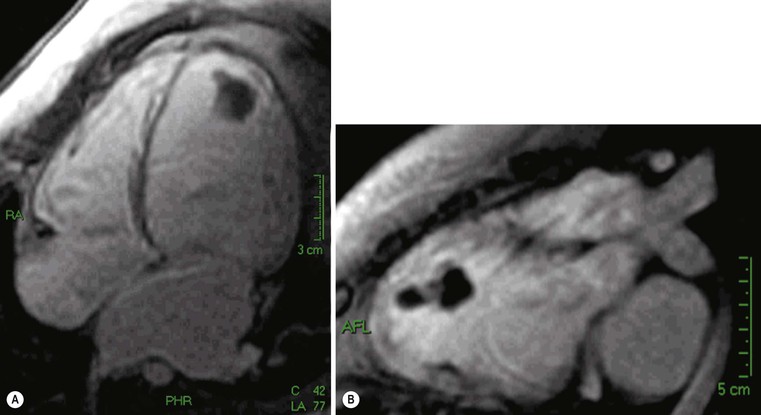
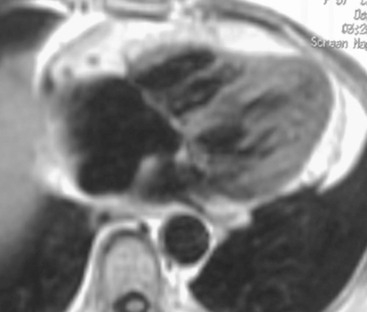
Chest X-ray has limited sensitivity, as cardiac contour abnormalities can be observed only in advanced stages; typically, on frontal view the third left cardiac arch is enlarged, heart size is increased and, eventually, there are signs of left atrium enlargement (carina widening and double contour of second right cardiac arch) (Fig. 21-8). However, the most relevant role of CXR is the evaluation of pulmonary vasculature; due to increased left ventricular end-diastolic pressure (LVEDP), left atrium, pulmonary veins and capillary wedge pressures increase, with consequent balanced distribution or caudocranial redistribution of pulmonary vessels. In the case of high pressure values, further evolution can cause pulmonary oedema (interstitial to alveolar). Echocardiography is considered the first-line examination in clinically suspected DCM; echocardiographic criteria are increased left ventricle end-diastolic diameter (Fig. 21-9), exceeding normal values of 112% after age and body surface area correction, ejection fraction lower than 45% and fractional shortening lower than 25%.17 Another useful parameter is the spherical index that correlates the left ventricular end-diastolic volume with the long-axis diameter and which is increased in DCM.18 Furthermore, echocardiography is able to demonstrate regional diffuse hypokinesis, sometimes restricted to apical segments, decreased forward flow velocities across all the valves and dominant E wave (early diastolic filling) at mitral flow interrogation, as well as complications such as mitral and/or tricuspid regurgitation and left ventricle thrombi.
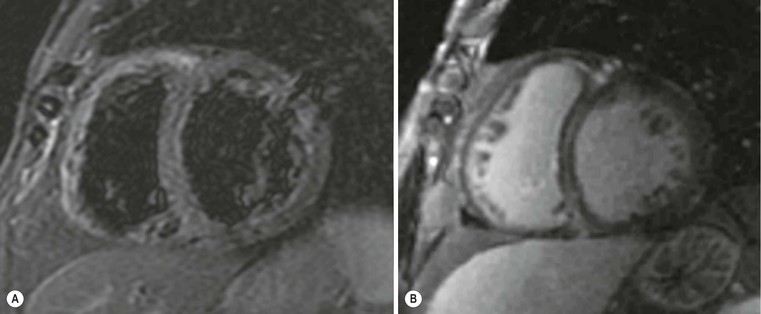
In the case of poor acoustic window, CMR is extremely useful, because of the high intrinsic contrast resolution between blood and myocardium (Fig. 21-10); planimetric and volume measurements are extremely accurate and more reproducible than echocardiographic ones,19 even if, in clinical settings, ultrasound measurements are commonly used. One of the major contributions of MRI is the differential diagnosis between ischaemic and non-ischaemic cardiomyopathy, which is crucial for decision making and treatment planning (revascularisation versus medical therapy and/or transplant); echocardiography, in fact, has a low specificity and thus differential diagnosis is not always easy. Using delayed enhancement technique, MRI is able to easily differentiate an ischaemic DCM (Fig. 21-11), demonstrating subendocardial or transmural scars, whereas non-ischaemic DCM late enhancement is absent or faint and limited to mesocardial layers, usually diffuse or septal (Fig. 21-12).20 Another important indication for MRI is the evaluation of the left ventricle before therapeutic procedures. In general, ECG and echocardiography with Doppler interrogation are used in clinical practice,21 but MRI is useful for assessing the presence of scar tissue in the inferior wall.22 Finally, MRI is extremely accurate in thrombus detection (Fig. 21-13); contrast-enhanced images show the highest sensitivity, also in areas where echocardiography has false negatives, e.g. close to the apex.23

Differential diagnosis of non-ischaemic forms is only partially feasible with delayed enhancement technique: in general, intramural or mesocardial enhancement is more frequent in post-myocarditis DCM than in the idiopathic form, but this must be further investigated and confirmed.8 Histology is still mandatory in these cases; in other secondary forms MRI is extremely useful, for example in dilated end stage HCM where the pattern and distribution of late enhancement help establish the correct diagnosis. The prognostic role of MRI in DCM is still under investigation; data are few and less robust than in HCM for risk assessment; left ventricular remodelling, ventricular tachycardia and sudden cardiac death seem to be related to the presence and amount of late enhancement.24
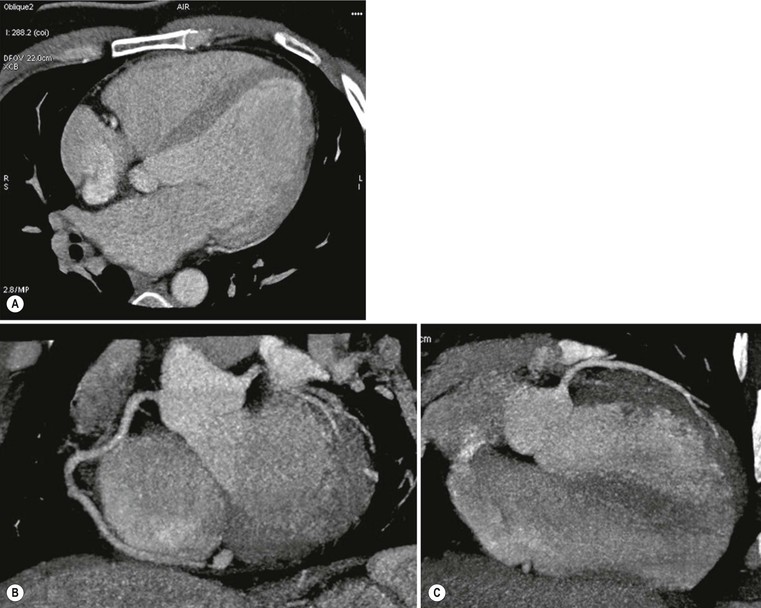
Cardiac CT can be used as an alternative to MRI in cases where echocardiography is difficult due to a poor acoustic window, to assess ventricular volumes and ejection fraction but, most importantly, it is useful in differential diagnosis between ischaemic and non-ischaemic forms, by means of its capability to exclude coronary artery disease (Fig. 21-14).25 CT is also highly accurate in left ventricle thrombus detection, while late enhancement technique does not seem to be useful due to lower contrast resolution compared with MRI.
Restrictive Phenotype
In this phenotype, the increased wall stiffness causes a rapid pressure increase with only a small volume increase; this restrictive pattern can occur in a wide range of diseases. The wall thickness is normal.2 Familial restrictive cardiomyopathy (RCM) is a very rare autosomal dominant disease; non-familial forms are caused by systemic disorders, such as amyloidosis, sarcoidosis, haemochromatosis, Anderson–Fabry disease, carcinoid heart disease, anthracycline toxicity, endomyocardial diseases, with or without hypereosinophilia (such as endomyocardial fibrosis) and endocardial fibroelastosis.
CXR is frequently negative; only at an advanced stage does left atrial enlargement become evident with signs of increased pulmonary venous pressure, as in mitral stenosis. Echocardiography often shows normal-sized ventricles, enlarged atria and normal or decreased contractile function; in some cases, such as in Loeffler’s syndrome, or endomyocardial fibroelastosis or carcinoid syndrome, endocardial thickening is evident (Fig. 21-15). Doppler evaluation of mitral flow is particularly important, showing elevated early diastolic velocity, short deceleration time and low and shortened atrial velocity.26 However, these abnormal parameters can be present also in the case of constrictive pericarditis, where pericardial stiffness does not allow ventricular filling; in this case, it is important to evaluate pericardial thickness, the interventricular septal kinetics and inferior vena cava flow during deep inspiration and expiration.26,27
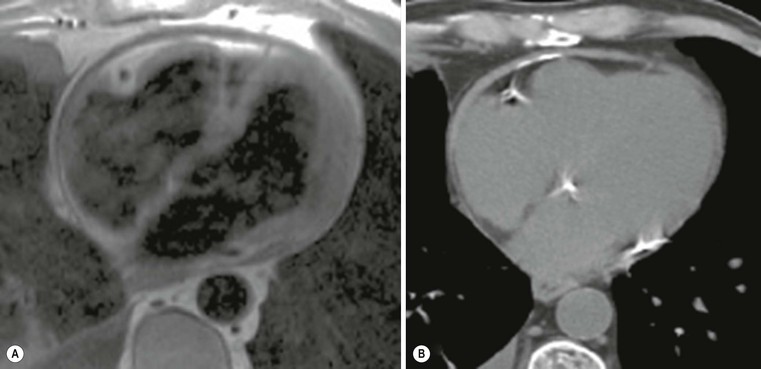
The pericardium is not easily assessed by ultrasound, and here cardiac CT or MRI can play a major role; a cut-off value of 4 mm is highly predictive of pericardial constriction,28 but it is important to remember that pericardial constriction without pericardial thickening can also occur. MRI can easily assess morphological and functional abnormalities of restriction (Fig. 21-16); T1- and T2-weighted (T1w, T2w) images can help in tissue characterisation (Fig. 21-17). As previously described, pre-contrast and delayed enhancement can be useful in differentiating some diseases, such as amyloidosis (Fig. 21-18), sarcoidosis and Anderson–Fabry disease (Fig. 21-7). Furthermore, cardiac MRI is extremely important in the assessment of iron overload of the myocardium (Fig. 21-19) as the measurement of T2* (transverse relaxation time in gradient-echo sequence) closely correlates with iron overload;29,30 consequently, it is possible to modulate chelation therapy in these patients, which has made it possible to reduce the mortality for heart failure, the leading cause of death in this disease. Cardiac CT, as well as MRI, can be used in differentiating RCM from constrictive pericarditis, by means of pericardium thickness measurement (Fig. 21-20); a potential advantage over MRI is the assessment of pericardial calcification.28
Arrhythmogenic Right Ventricular Cardiomyopathy
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a relatively uncommon familial disease, usually autosomal dominant, characterised by various desmosome protein mutations. This results in right ventricle dysfunction, global or regional, with or without left ventricle involvement, in the presence of histopathological evidence of right ventricular myocardial replacement with fatty and fibrous tissue in various amounts. There is preferential localisation in the so-called triangle of dysplasia (outflow tract, inflow tract and apex). There are distinctive electrocardiographic abnormalities, according to previously published and recently revisited criteria.31,32
ARVC is a frequent cause of sudden cardiac death in young people, due to severe ventricular arrhythmias. For this reason, early diagnosis is crucial, based on the combination of many criteria proposed in 1994: familial history, ECG alterations, repolarisation abnormalities, arrhythmias (ventricular tachycardia with left bundle branch block, extrasystoles >1000/24 h), right ventricle systolic dysfunction, fibro-fatty replacement at endomyocardial biopsy. Based on the severity of the alterations, all these criteria are distinguished in major and minor; from their combination, a diagnosis of ARVC can be established, or excluded in their absence. In cases of doubt, it is important to regularly monitor the patient.
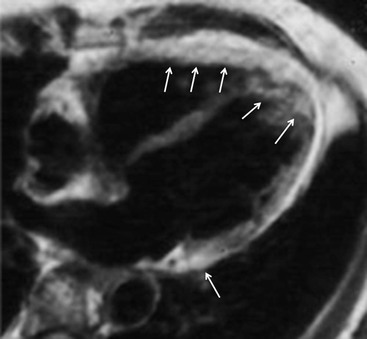
In 2010 these criteria were revisited and, particularly, quantitative parameters for right ventricle enlargement and ejection fraction were introduced, by means of ultrasound or cardiac MRI measurements, aimed to increase sensitivity (Table 21-3 and Fig. 21-21).2 MRI has been used in the past quite exclusively to demonstrate fatty infiltration of the right ventricle myocardium, particularly in the so-called triangle of dysplasia, the region contained between the subtricuspid area, the apex and the free wall of the outflow tract. However, the capability of assessing the presence of fat is strictly related to the amount of the fat, because of the limited spatial resolution: for this reason it is possible to recognise only cases with extensive replacement (Fig. 21-22). Another issue is the limitation of intramyocardial versus subepicardial differentiation of fat accumulation, partly because of limited spatial resolution but also because subepicardial fat accumulation can be recognised in other physiological and pathological conditions, such as obesity, steroid therapy and old age (Fig. 21-23).
TABLE 21-3
European Society of Cardiology Classification of Secondary Cardiomyopathies
| Secondary Cardiomyopathies | |

Modified from Elliott et al.2
In the revised criteria, MRI has been included only for the assessment of ventricular size and global/segmental functional assessment, as it has been demonstrated to be more sensitive than ultrasound for right ventricular systolic function evaluation; it is unique in volume measurements, as it uses a 3D approach (unlike ultrasound) that needs a geometrical assumption (not available for the right ventricle) for measuring volumes. Also, regional function is better evaluated by MRI: systolic bulging and aneurysms of the right ventricle anterior wall are easily detected (Fig. 21-24), also in the free wall, not always assessable by echocardiography.
Recent data have been published on the use of delayed enhancement technique to demonstrate the presence of fibrotic tissue33 although this is not easy to visualise in the thin myocardium of the right ventricle. Cardiac CT is only relatively helpful, considering the wide role of MRI; however, in small series, CT has detected small foci of fat deposition/substitution even in the right ventricle, due to its high spatial resolution and good contrast resolution for fat.34 However, CT is less suited to assessing regional wall motion abnormalities of the right ventricle.
Unclassified Cardiomyopathy
In this group two entities are included: left ventricular non-compaction and takotsubo cardiomyopathy. Left ventricular non-compaction (LVNC) is characterised by prominent left ventricular trabeculae and deep intertrabeculae recesses;35 this results in segments of thickened myocardium, composed of thin compacted epicardium with a thick endocardial layer. It is unclear whether LVNC is a separate CMP or a congenital or acquired trait shared by other phenotypes; it can be isolated or associated with congenital anomalies (complex cyanotic, Ebstein) or muscular dystrophies. It is frequently familial, with a wide range of abnormalities seen in about 25% of asymptomatic relatives.
Echocardiography is the first and often only diagnostic tool for assessing LVNC, especially in paediatric patients. Increased trabeculation is usually detected in mid-apical segments, on both lateral wall and septum (Fig. 21-25), with the latter normally being non-trabeculated. In difficult cases, MRI can be useful, using a cut-off value of 2.3 for the ratio between non-compacted and compacted myocardium (Fig. 21-26).36 Furthermore, delayed enhancement is able to demonstrate the presence of fibrotic tissue in the compacted myocardium, more often in the forms associated with muscular dystrophies (Fig. 21-27).
Takotsubo CMP or transient left ventricular apical ballooning syndrome is characterised by a reversible regional systolic dysfunction, associated with chest pain and negative invasive coronary angiography; typical presentation is an acute coronary syndrome, more in post-menopausal women after a physical or emotional stress, with noradrenaline acting as a neuromediator.37 Functional recovery usually occurs within days or a couple of weeks.
Echocardiography is usually able to detect the abnormal function and the ballooning,38 but, because of the acute presentation, invasive coronary angiography is required to exclude obstructive coronary artery disease, according to guidelines. In these cases, after a negative coronary angiography, there is a strong indication for CMR, with the aim to differentiate a myocardial infarction with normal coronary arteries from an acute myocarditis and a takotsubo CMP.39 CMR is indeed able to detect the functional ballooning, but, more importantly, the oedema of the myocardium without any delayed enhancement, indicating the absence of necrosis and the reversibility of the damage (Fig. 21-28).40
Myocarditis
Myocarditis is not included in the ESC CMP classification as a single definite category, but is included in the acquired group as inflammatory CMP; in the AHA classification it is cited in the dilated phenotype for chronic myocarditis, while acute myocarditis does not show a precise phenotype. Consequently, myocarditis must be regarded separately from the classification, also when taking into consideration its increasing incidence and the recently increased possibilities to define the diagnosis.
Myocarditis is an acute or chronic inflammatory process affecting the myocardium, toxic or infective (viral, bacterial, rickettsial, fungal, parasitic), or due to hypersensitivity reactions.1 It typically evolves through active healing and healed stages, with progression of inflammatory infiltrates, interstitial oedema, myocyte necrosis and finally scarring; in some cases, subclinical forms of viral myocarditis can trigger an autoimmune reaction causing immunological damage to the myocardium and/or cytoskeletal disruption, leading to a DCM phenotype with LV dysfunction. In these cases, viral persistence and chronic inflammatory infiltrates have been demonstrated.
Histology obtained through an endomyocardial biopsy is still considered the gold standard for the diagnosis,41 based on the combination of leucocyte infiltration and necrosis defined by the so-called Dallas criteria,42 though these criteria have been recently debated. Substantial refinement of the diagnosis can been reached by using molecular analysis of the specimens, such as DNA–RNA extraction and polymerase chain reaction.43 Acute presentation can often mimic an acute coronary syndrome, with chest pain exertion dyspnoea, ECG abnormalities and mild enzyme elevation.39,44 In this case there is an indication for invasive coronary angiography to exclude obstructive coronary artery disease, even if the presence of a recent viral infection or unexplained fever can indicate the correct diagnosis.
Echocardiography is usually performed to assess and quantify left ventricle systolic dysfunction; in the case of associated pericardial effusion, ultrasound can suggest the suspicion of an inflammatory process, but there is need for further examination. In the acute presentation, after negative coronary angiography, there is a strong indication to perform CMR, which can demonstrate oedema and delayed enhancement in the subepicardial layer, most frequently in the lateral and/or inferior wall (Fig. 21-29). This pattern easily allows differential diagnosis from acute myocardial infarction, where delayed enhancement is located in the subendocardial layer or is transmural, and from takotsubo CMP, where delayed enhancement is typically absent. However, a negative delayed enhancement study does not exclude an acute myocarditis, as there is not always macroscopic detectable necrosis; in this case it is important to acquire MR images before and immediately after administration of contrast agent (early enhancement), in order to demonstrate inflammatory hyperaemia. The combination of oedema imaging (T2w sequences), basal, early and late enhancement imaging constitutes the cornerstone for the diagnosis, according to the so-called Lake-Louise criteria.45 CMR is also useful and accurate for evaluating biventricular global and regional systolic function and eventually associated pericardial effusion, with or without pericardial enhancement, in the case of myopericarditis. Acute fulminant forms or cardiogenic shock can represent other rare but possible acute presentations.
Other clinical presentations can be more subtle, such as new-onset cardiac failure or tachyarrhythmias; these presentations are more often associated with chronic myocarditis. In these situations, echocardiography is useful for excluding other diseases: for example, a new onset of heart failure. In chronic myocarditis, MRI with late enhancement technique demonstrates fibrosis, typically located in the mesocardium.46 Enhancement is generally less intense than in the acute forms, diffuse rather than patchy and located in the lateral wall or in the septum, the latter being more frequent in tachyarrhythmias (Fig. 21-30).
Valvular Heart Disease
Contrary to previous reports in the literature, the aetiology of acquired valve disease is now considered as being usually degenerative, rather than due to rheumatic or infective causes; the prevalence of mitral regurgitation and aortic stenosis is higher than that of mitral stenosis and aortic insufficiency. Also the age of onset is increasing. Radiographic findings are often minimal and non-specific, and the assessment is based on echocardiography.47
Mitral Valve Disease
In Western countries, non-rheumatic mitral valve disease is the most common manifestation of mitral disease; in non-Western countries, rheumatic heart disease is still quite prevalent. Among non-rheumatic diseases, mitral regurgitation is the most common, while non-rheumatic mitral stenosis is very rare. Many conditions can result in significant mitral regurgitation, affecting mitral leaflets (prolapse, endocarditis, mucopolysaccharidosis, lupus, rheumatoid arthritis) or subvalvular apparatus (annular dilatation, chordae tendineae rupture, annular calcification, myocardial infarction, hypertrophic cardiomyopathy). In mitral regurgitation, a portion of the left ventricular stroke volume is directed retrogradely into the atrium during systole, returning to the left ventricle during diastole, with consequent left ventricular volume loading. To maintain an adequate stroke volume both the left ventricular stroke volume and the ejection fraction increase.
Acute regurgitation may result from infective endocarditis or rupture of chordae tendineae/papillary muscles. Sudden volume loading into the non-compliant left atrium may result in markedly elevated atrial pressure, causing acute pulmonary oedema and symptoms of heart failure.48 Rupture or elongation of chordae tendineae, ischaemic and non-ischaemic cardiomyopathy, hypertrophic obstructive cardiomyopathy and rheumatic heart disease are all causes of chronic mitral regurgitation.48 In response to the chronic volume load both the left atrium and left ventricle dilate, thus serving as a reservoir for the regurgitant volume without necessarily increasing pulmonary vascular pressure. However, if the left ventricle decompensates and the forward stroke volume decreases, heart failure may arise.48
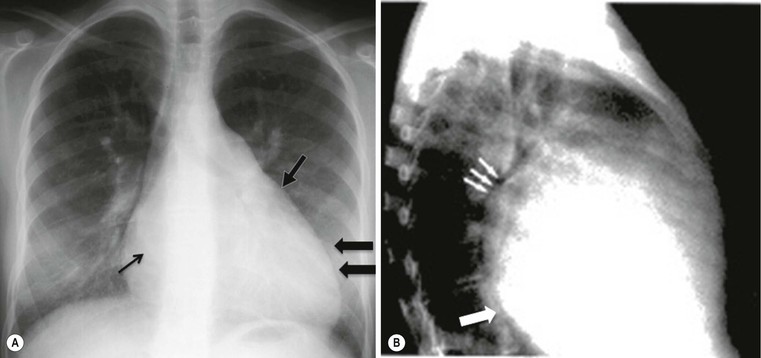
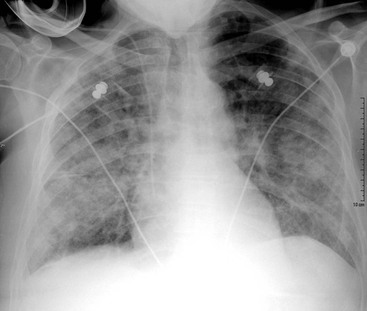
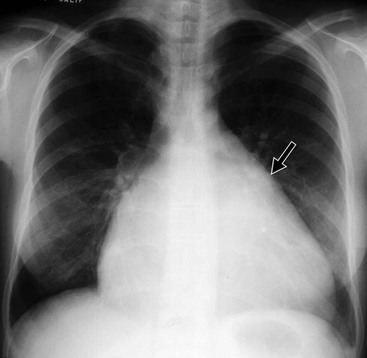
The appearances on CXR depend on the duration and severity of the mitral regurgitation and any other associated heart disease. Acute, severe mitral regurgitation may present with pulmonary oedema, but with a virtually normal heart size and shape (Fig. 21-31). After an interval, the heart usually decompensates by developing left ventricular dilatation, which may be marked (Fig. 21-32). Selective left atrial enlargement may be absent, slight or moderate, with or without left atrial appendage enlargement (Fig. 21-32). Pulmonary vascular changes reflect the haemodynamic derangement and the effects of treatment.
As previously described, mitral regurgitation can have many different causes such as mitral prolapse or chordae tendinee rupturae (during bacterial endocarditis, collagen diseases, acute myocardial infarction) or it may be functional (DCM, ischaemic).
Mitral Valve Prolapse
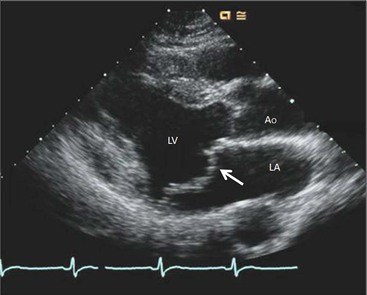
The most common cause of severe (non-ischaemic) mitral regurgitation is the mitral valve prolapse syndrome.49 Mitral valve prolapse is defined as systolic bowing of the mitral leaflet more than 2 mm beyond the annular plane into the atrium,48 caused by rupture or elongation of the chordae tendineae. The middle scallop of the posterior leaflet is the most often affected.48 Mitral valve prolapse is due to elongation of the chordae tendineae associated with myxomatous degeneration of the valve leaflets, occurring alone or in association with Marfan’s syndrome and in patients with atrial septal defect. The diagnosis is typically made by echocardiography (Fig. 21-33), but it is also possible on CMR, with two- and three-chamber views preferred; associated imaging findings include leaflet thickening (thickness of >5 mm) and flail leaflet.48 Cardiac CT also has good sensitivity for detecting mitral valve prolapse, small vegetations and ruptured chordae; furthermore, cardiac CT is useful for assessing valvular and subvalvular calcifications50 and is increasingly used in valvular assessment. Prolapse of the mitral valve may be associated with chest pain and ECG changes, which may suggest ischaemic heart disease.
Chordal Rupture
Chordal rupture may complicate bacterial endocarditis, or less frequently myocardial infarction or connective tissue diseases, leading to flail of part of a leaflet; this is the eversion of the mitral leaflet tip into the atrium during systole,48 preventing proper closure in systole and producing severe mitral regurgitation. In the case of an acute event, acute pulmonary oedema is seen on CXR, usually without cardiomegaly (Fig. 21-31).
Functional Mitral Regurgitation
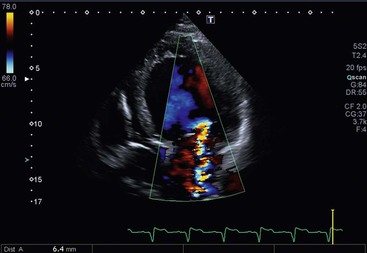
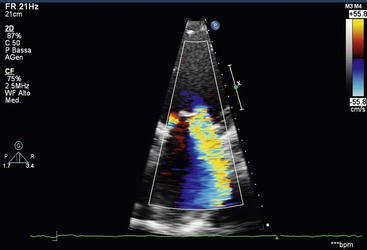
Functional mitral regurgitation may occur in dilated cardiomyopathy or ischaemic cardiac failure. In this case the valve is normal, but regional wall motion abnormalities, left ventricular dilatation, tethering of chordae tendineae or annular dilatation, alone or in combination, lead to dysfunction of the mitral apparatus with ‘functional’ mitral regurgitation.48 Mitral regurgitation in these situations is very common, but severe (Fig. 21-34). Mitral regurgitation detection and severity are usually assessed by 2D Doppler echocardiography, which can detect left atrial dilatation, increased atrial emptying volume and gradual closure of aortic valve during systole, coupled with visualisation of systolic regurgitant colour-coded flow within the left atrium. Pulsed Doppler interrogation is extremely sensitive also to small amounts of regurgitant flow; the extent of penetration and the area of the regurgitant jet within the left atrium allow for a semiquantitative estimation of the disease (mild, moderate, severe) (Fig. 21-35). However, in the case of asymmetrical jets (i.e. flail), this method underestimates the severity. Furthermore, continuous Doppler is very sensitive, giving a characteristic high-velocity, parabolic, systolic spectrum of the flow.
MRI identifies mitral regurgitation in cine as a retrograde jet through the mitral orifice from the left ventricle into the left atrium, due to turbulent flow and consequent spin dephasing (Fig. 21-36). Mitral valve regurgitation can be quantified as the difference between left and right ventricle stroke volumes (LVSV and RVSV). Every difference between the two measured stroke volumes indicates the amount of blood which comes back through the insufficient valve during diastole.51 This estimation is valid only if the tricuspid valve is competent (tricuspid regurgitation is reported in up to 50% of patients with significant mitral regurgitation); moreover, the calculation of RVSV is less reproducible than LVSV due to the extensive trabeculation of the right ventricle.51 In the case of combined aortic and mitral regurgitation, the difference represents the sum of regurgitant volumes.51
Phase contrast MRI can discriminate between antegrade and retrograde flow during the cardiac cycle; the mitral regurgitant volume (MRV) can be measured by MRI as the difference between the LVSV and the aortic forward flow. The regurgitant fraction (RF) is the ratio of the MRV divided by the LVSV.51 It may also be possible to directly measure mitral flow by phase contrast velocity flow mapping at the tips of the mitral valve leaflets but this is better obtained with a specialised imaging sequence which tracks the motion of the mitral valve annulus during the cardiac cycle to adjust the plane of velocity encoding for diastolic mitral valve motion.49,51,52 With cine-phase contrast MRI, mitral regurgitation can be calculated as left ventricular inflow through the mitral valve minus left ventricular outflow in the ascending aorta.49 This approach is also applicable in patients with mitral and aortic regurgitation, since diastolic left ventricular inflow (= left ventricular mitral inflow and aortic regurgitation volume) is equal to the systolic left ventricular outflow (= aortic outflow and mitral regurgitant volume).49
The American Heart Association and the American College of Cardiology53,54 have established echocardiographic criteria for grading the severity of mitral regurgitation. Even in the absence of inter-society established criteria for MRI, the measurements of regurgitant volume (RV) and fraction derived from LV volume and ascending aortic flow allow grading of regurgitation as:48
For a comprehensive assessment of patients with mitral regurgitation, three components are mandatory: (1) quantification of regurgitation, (2) assessment of left ventricular adaptation to volume overload and (3) anatomy of mitral valve and subvalvular apparatus. Whereas MRI satisfies (1) and (2), the method of choice for assessing valve anatomy is echocardiography, although improvements in MR imaging strategies allow detection of morphological abnormalities such as flail mitral valve leaflets.48
Mitral Stenosis
Mitral stenosis is a structural abnormality of the mitral valve that prevents proper opening during diastolic filling of the left ventricle. Increased left atrial pressure is necessary to move blood across the stenotic mitral valve and into the left ventricle. Chronic elevation of left atrial pressure causes atrial dilatation and pulmonary venous hypertension. Atrial fibrillation (due to atrial dilatation) and dyspnoea (due to pulmonary venous hypertension) are common symptoms of mitral stenosis. Prolonged pulmonary venous hypertension may also lead to right ventricular dilatation and failure, as well as to tricuspid regurgitation.48
Mitral stenosis (MS) is highly prevalent in developing countries because of its association with rheumatic fever, but is also seen in developed countries.55
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree